Advertisements
Advertisements
Question
Draw the structure of boric acid showing hydrogen bonding. Which species is present in water? What is the hybridisation of boron in this species?
Solution
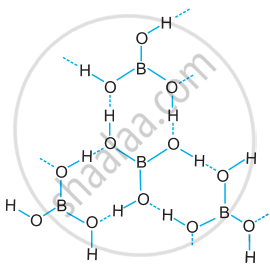
Structure of boric acid; the dotted lines represent hydrogen bonds
[B(OH)4]– units are present in water. Boron has sp3 hybridisation in [B(OH)4]– unit.
APPEARS IN
RELATED QUESTIONS
Explain what happens when boric acid is heated.
Explain the following reaction.
Hydrated alumina is treated with aqueous NaOH solution.
A certain salt X, gives the following results.
- Its aqueous solution is alkaline to litmus.
- It swells up to a glassy material Y on strong heating.
- When conc. H2SO4 is added to a hot solution of X, white crystal of an acid Z separates out.
Write equations for all the above reactions and identify X, Y and Z.
Write a balanced equation for \[\ce{H3BO3 ->[\Delta]}\]?
Boric acid is polymeric due to ______.
An aqueous solution of borax is _______.
Draw the structures of BCl3.NH3 and AlCl3 (dimer).
A compound (A) of boron reacts with NMe3 to give an adduct (B) which on hydrolysis gives a compound (C) and hydrogen gas. Compound (C) is an acid. Identify the compounds A, B and C. Give the reactions involved.
Diborane (B2H6) reacts independently with O2 and H2O to produce, respectively:
Boron reacts with nitric acid to form ______.
