Advertisements
Advertisements
Question
Explain the following with suitable examples: Paramagnetism
Solution
Paramagnetism: The substances that are attracted by a magnetic field are called paramagnetic substances. Some examples of paramagnetic substances are O2, Cu2t, Fe3t, and Cr3t.
Paramagnetic substances get magnetised in a magnetic field in the same direction, but lose magnetism when the magnetic field is removed. To undergo paramagnetism, a substance must have one or more unpaired electrons. This is because the unpaired electrons are attracted by a magnetic field, thereby causing paramagnetism.
APPEARS IN
RELATED QUESTIONS
What is ferromagnetism?
What type of magnetism is shown in the following alignment of magnetic moments?

What type of magnetism is shown by a substance if magnetic moments of domains are arranged in same direction?
Explain the following with suitable examples: Antiferromagnetism
Give reasons:Ferrimagnetic substances show better magnetism than antiferromagnetic substances.
Explain why:
(i) Transition elements form coloured compounds.
(ii) Interhalogen compounds are more reactive than their constituent elements.
(iii) Cu+ is diamagnetic but Cu2+ is paramagnetic. (Z = 29)
Out of [CoF6]3- and [Co(en)3]3+, which one complex is
(i) paramagnetic
(ii) more stable
(iii) inner orbital complex and
(iv) high spin complex
(Atomic no. of Co = 27)
The BH curve for a ferromagnetic material is shown in the figure. The material is placed inside a long solenoid which contains 1000 turns/cm. The current that should be passed in the solenonid to demagnetize the ferromagnet completely is
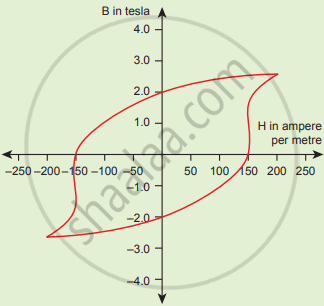
What is magnetic susceptibility?
What happens to the domains in a ferromagnetic material in the presence of external magnetic field?
Substances which are weakly repelled in magnetic field are known as ____________.
All those atoms or molecules which have an odd number of electrons are
When heated to high temperature, ferromagnetic substance changes to ____________.
Which type of substances would make better permanent magnets?
Which one of the following would feel attraction when placed in magnetic field: Co2+, Ag+, Ti4+, Zn2+
The correct order of bond strength is ______.
The susceptibility of a paramagnetic material is 99. The permeability of the material in Wb/A-m is ______.
[permeability of the free space μ0 = 4π × 10-7 Wb/A - m]
Which one of the following compounds is diamagnetic and colourless?
