Advertisements
Advertisements
Question
Explain the following reaction with the balanced equation.
Sodium burns in air
Solution
On burning, sodium metal combines with oxygen in the air to form sodium oxide.
\[\ce{\underset{\text{Sodium}}{4Na_{(s)}} + \underset{\text{Oxygen}}{O_{2(g)}} -> \underset{\text{Sodium oxide}}{2Na2O_{(s)}}}\]
APPEARS IN
RELATED QUESTIONS
Give one example each of which illustrates the following characteristics of a chemical reaction:
evolution of a gas
What do you observe when silver nitrate is added to a solution of sodium chloride?
Fill in the blank
When a piece of copper is added to silver nitrate solution, it turns ............in colour.
Divide the metals Cu, Zn, Ca, Mg, Fe, Na, Li into three groups, namely reactive metals, moderately reactive metals and less reactive metals.
Give a balanced equation for the reversible catalytic reaction involving nitrogen as one of the reactants.
Select the correct answer for the statement given below:
A neutral oxide which does not react with an acid or a base to give salt and water.
Complete the statement by filling in the blank with the correct word:
The metal which reacts with steam and the reaction is reversible is ________.
Give a balanced equation for the following type of reaction:
A displacement reaction in which a metal above hydrogen in the reactivity series, displaces another metal from the solution of its compound.
Classify the following metals based on their reactivity.
Cu, Zn, Ca, Mg, Fe, Na, Li, Hg
| More reactive | Moderately reactive | Less reactive |
Explain the following reaction with the balanced equation.
Reaction of aluminium with oxygen
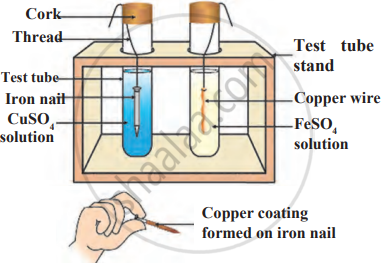
Observe the following diagram and identify the type of reaction and write observation.
Which of the following is the correct arrangement of the given metals in ascending order of their reactivity?
Zinc, Iron, Magnesium, Sodium
Compound X and aluminium are used to join railway tracks.
- Identify the compound X
- Name the reaction
- Write down its reaction.
A metal A, which is used in thermite process, when heated with oxygen gives an oxide B, which is amphoteric in nature. Identify A and B. Write down the reactions of oxide B with HCl and NaOH.
Explain the following
- Reactivity of Al decreases if it is dipped in HNO3
- Carbon cannot reduce the oxides of Na or Mg
- NaCl is not a conductor of electricity in solid state whereas it does conduct electricity in aqueous solution as well as in molten state
- Iron articles are galvanised.
- Metals like Na, K, Ca and Mg are never found in their free state in nature.
Metal ‘A’ has electronic configuration 2, 8, 1 and metal ‘B’ has electronic configuration 2, 8, 8, 2. Out of these, which metal is more reactive? Write the reaction of this metal with dilute HCl acid.
Arrange the following as per the instruction given in the bracket:
Al, K, Mg, Ca (decreasing order of its reactivity)
