Advertisements
Advertisements
Question
Give one test to distinguish between the following pair of chemicals.
Sodium nitrate solution and sodium chloride solution.
Solution
Sodium chloride solution and sodium nitrate solution can be distinguished by using conc. Sulphuric acid to the salt solution, add freshly prepared ferrous sulphate solution and pour a few drops of conc. H2SO4 along the sides of the tube. If it's sodium nitrate solution then a brown ring would appear at the junction of the two liquid layers. But if its sodium chloride solution, it would not undergo any visible reaction.
APPEARS IN
RELATED QUESTIONS
Certain blank spaces are left in the following table and these are labelled as A, B, C, D
and E. Identify each of them
| Lab preparation of | Reactants used | Products formed | Drying Agent | Method of collection |
|
| 1 | HCl gas | NaCl + H2SO4 |  |
conc. H2SO4 | 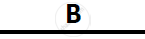 |
| 2 | NH3 gas |
|
Mg(OH)2 NH3 |
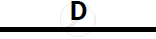 |
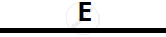 |
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
State a safety precaution you would take during the preparation of hydrochloric acid.
Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following.
- Name the acid used. Why is this particular acid preferred to other acids?
- Give the balanced equation for the reaction.
- Name the drying agent used in drying hydrogen chloride gas.
- Phosphorous pentoxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gas. Why?
- Why is the direct absorption of \[\ce{HCl}\] gas in water not feasible?
- What arrangement is done to dissolve \[\ce{HCl}\] gas in the water?
Give the balanced equation for the laboratory preparation of hydrogen chloride gas reaction.
Explain why a solution of hydrogen chloride in water turns blue litmus red and conducts electricity, while a solution of the same gas in toluene:
- has no effect on litmus, and
- does not conduct electricity.
Explain why dry hydrogen chloride gas does not affect a dry strip of blue litmus paper but it turns red in the presence of a drop of water.
The drying agent used to dry \[\ce{HCl}\] gas is ______.
How will you prove that the gas prepared is HCI?
What are the important precautions?
Explain, why (or give reasons for)
Hydrogen chloride is not collected over water.
Describe an experiment to prove the following:
HCI gas contains the element chlorine.
Write the equation for:
The reaction between hydrogen chloride and ammonia.
State the observation for action of dilute hydrochloiric acid or iron (II) sulphate.
One chemical test that would enable you to distinguish between the following pair of chemicals. Describe what happens with each chemical or state 'no visible reaction'.
Sodium chloride solution and sodium nitrate solution.
One chemical test that would enable you to distinguish between the following pair of chemicals. Describe what happens with each chemical or state 'no visible reaction'.
Sodium sulphate solution and sodium chloride solution.
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
Identify the gas evolved in the following reaction:
\[\ce{MnO2}\] reacts with concentrated \[\ce{HCl}\].
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
