Advertisements
Advertisements
Question
Give the reaction of glucose with hydrogen cyanide. Presence of which group is confirmed by this reaction?
Solution
The reaction of glucose with hydrogen cyanide:
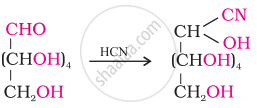
This reaction confirms the presence of the carbonyl group (>C = O) in glucose.
APPEARS IN
RELATED QUESTIONS
Glucose on reaction with HI gives n-hexane. What does it suggest about the structure of glucose?
Write the reactions involved when D-glucose is treated with the following reagent:
Br2 water
Choose the appropriate answer(s) for the below representation from the options given
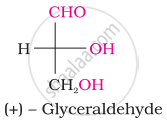
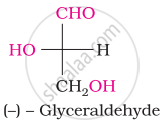
Acetylation of glucose yields ____________.
Which of the following statements is incorrect regarding glucose?
Reduction of glucose by HI suggest that ____________.
The α-D glucose and β-D glucose differ from each other due to difference in carbon atom with respect to its ____________.
The two forms of D-glucopyranose obtained from the solution of D-glucose are called ____________.
The number of chiral carbons in ß-D(+) glucose is ____________.
In the following reaction, identify A and B:
\[\begin{array}{cc}
\ce{C6H12O6 ->[Acetic anhydride] A}\\
\downarrow \text{Conc. nitric acid}\phantom{...}\\
\ce{B}\phantom{.................}\end{array}\]
