Advertisements
Advertisements
Question
α-Helix is a secondary structure of proteins formed by twisting of polypeptide chain into right-handed screw like structures. Which type of interactions are responsible for making the α-helix structure stable?
Solution
In α-helix structure of protein, a polypeptide chain is stabilize by the formation of intramolecular H – bonding between – NH – group of amino acids in one turn with the >C = O groups of amino acids belonging to adjacent turn.
APPEARS IN
RELATED QUESTIONS
How is tripeptide formed?
What type of bonding helps in stabilising the ∝-helix structure of proteins?
The helical structure of protein is stabilized by:
Proteins are found to have two different types of secondary structures viz. α-helix and β-pleated sheet structure. α-helix structure of protein is stabilised by:
Optical rotations of some compounds along with their structures are given below which of them have D configuration.
| (I) | 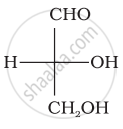 |
| (II) | 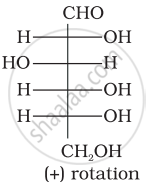 |
| (III) | 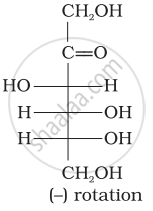 |
Proteins can be classified into two types on the basis of their molecular shape i.e., fibrous proteins and globular proteins. Examples of globular proteins are:
(i) Insulin
(ii) Keratin
(iii) Albumin
(iv) Myosin
Which of the following are purine bases?
(i) Guanine
(ii) Adenine
(iii) Thymine
(iv) Uracil
Protein found in a biological system with a unique three-dimensional structure and biological activity is called a native protein. When a protein in its native form, is subjected to a physical change like change in temperature or a chemical change like, change in pH, denaturation of protein takes place. Explain the cause.
The total number of negative charge in the tetrapeptide, Gly-Glu-Asp-Tyr at pH 12.5 will be ______. (Integer answers)
β-pleated sheet structure in proteins refers to ______.
