Advertisements
Advertisements
Question
Match the items given in Column I with the items given in Column II.
| Column I | Column II |
| (i) Mg in solid state | (a) p-Type semiconductor |
| (ii) MgCl2 in molten state | (b) n-Type semiconductor |
| (iii) Silicon with phosphorus | (c) Electrolytic conductors |
| (iv) Germanium with boron | (d) Electronic conductors |
Solution
| Column I | Column II |
| (i) Mg in solid state | (d) Electronic conductors |
| (ii) MgCl2 in molten state | (c) Electrolytic conductors |
| (iii) Silicon with phosphorus | (b) n-Type semiconductor |
| (iv) Germanium with boron | (a) p-Type semiconductor |
Explanation:
(i) Mg in solid state show electronic conductivity due to presence of free electrons hence, they are known as electronic conductors.
(ii) MgCl2 in molten state show electrolytic conductivity due to presence of electrolytes in molten state.
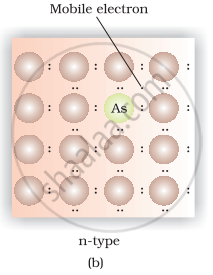
(iii) Silicon doped with phosphorus contain one extra electron due to which it shows conductivity under the influence of electric field and known as p-type semiconductor.
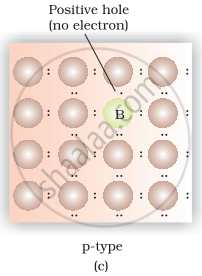
(iv) Germanium doped with boron contains one hole due to which it shows conductivity under the influence of electric field and known as n-type semiconductor.
APPEARS IN
RELATED QUESTIONS
Classify the following solids in different categories based on the nature of intermolecular forces operating in them:
Potassium sulphate, tin, benzene, urea, ammonia, water, zinc sulphide, graphite, rubidium, argon, silicon carbide.
Classify each of the following solids as ionic, metallic, molecular, network (covalent) or amorphous.
(i) Tetra phosphorus decoxide (P4O10)
(ii) Ammonium phosphate (NH4)3PO4
(iii) SiC
(iv) I2
(v) P4
(vii) Graphite
(viii) Brass
(ix) Rb
(x) LiBr
(xi) Si
Based on the nature of intermolecular forces, classify the following solids:
Sodium sulphate, Hydrogen
The existence of a substance in more than one solid modifications is known as ____________.
Which of the following is not a characteristic property of solids?
Which of the following is not a characteristic of a crystalline solid?
Which of the following conditions favours the existence of a substance in the solid state?
Which among the following will show anisotropy?
