Advertisements
Advertisements
Question
Match the type of packing given in Column I with the items given in Column II.
| Column I | Column II |
| (i) Square close packing in two dimensions |
(a) Triangular voids |
| (ii) Hexagonal close packing in two dimensions |
(b) Pattern of spheres is repeated in every fourth layer |
| (iii) Hexagonal close packing in three dimensions |
(c) Coordination number 4 |
| (iv) Cubic close packing in three dimensions |
(d) Pattern of sphere is repeated in alternate layers |
Solution
| Column I | Column II |
| (i) Square close packing in two dimensions |
(c) Coordination number 4 |
| (ii) Hexagonal close packing in two dimensions |
(a) Triangular voids |
| (iii) Hexagonal close packing in three dimensions |
(d) Pattern of sphere is repeated in alternate layers |
| (iv) Cubic close packing in three dimensions |
(b) Pattern of spheres is repeated in every fourth layer |
Explanation:
(i) Square close packing in two dimensions each sphere have coordination number 4, as shown below.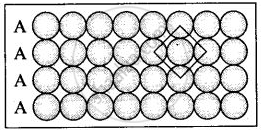
(ii) Hexagonal close packing in two dimensions each sphere has coordination number 6 as shown below and creates a triangular void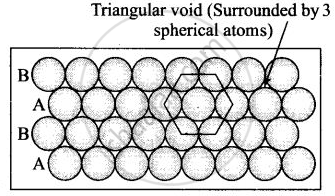
(iii) Hexagonal close packing in 3 dimensions is a repeated pattern of sphere in alternate layers also known as ABAB pattern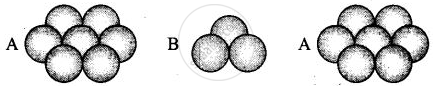
(iv) Cubic close packing in a 3 dimensions is a repeating pattern of sphere in every fourth layer.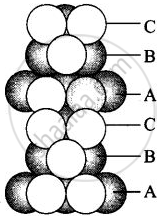
APPEARS IN
RELATED QUESTIONS
What is the two dimensional coordination number of a molecule in square close packed layer?
A compound is formed by two elements M and N. The element N forms ccp and atoms of M occupy 1/3rdof tetrahedral voids. What is the formula of the compound?
Hexagonal close packed arrangement of ions is described as ____________.
In NaCl structure ____________.
How can you best describe the elongated octahedral structure of blue vitriol, CuSO4.5H2O?
A compound forms hexagonal close-packed structure. What is the total number of voids in 0.5 mol of it? How many of these are tetrahedral voids?
The number of tetrahedral and octahedral voids in a CCP array of 100 atoms are respectively:
The right option for the number of tetrahedral and octahedral voids in the hexagonal primitive unit cell is _______.
The right options for the number of tetrahedral and octahedral voids in the hexagonal primitive unit cells is ______.
Ionic radii of cation A+ and anion B- are 102 and 181 pm respectively. These ions are allowed to crystallize into an ionic solid. This crystal has cubic close packing for B-. A+ is present in all octahedral voids. The edge length of the unit cell of the crystal AB is ______ pm. (Nearest integer)
