Advertisements
Advertisements
Question
Name the reagent used to bring about the following conversion.
Ethyl bromide to ethyl isocyanide
Solution
| Reactant | Reagent | Product |
| Ethyl bromide | \[\ce{->[Alcohol][AgCN]}\] | Ethyl isocyanide |
APPEARS IN
RELATED QUESTIONS
from the following pair would undergo SN2 faster from the other?
a. ![]() b.
b. ![]()
Name the reagent used to bring about the following conversion.
1-Chloropropane to 1-nitropropane
Convert the following:
Benzyl alcohol to benzyl cyanide
HCl is added to a hydrocarbon ‘A’ \[\ce{(C4H8)}\] to give a compound ‘B’ which on hydrolysis with aqueous alkali forms tertiary alcohol ‘C’ \[\ce{(C4H10O)}\]. Identify ‘A’ ,‘B’ and ‘C’.
Observe the following and answer the question given below.

Comment on the bond length of C–X bond in it.
Propane nitrile can be prepared by heating ____________
Write the correct order of increasing ease of dehydrohalogenation.
\[\ce{\underset{\text{(I)}}{CH3 - CH2 - CH2 - Cl}}\]
\[\ce{\underset{\text{(II)}}{CH3 - CH(Cl) - CH3}}\]
\[\begin{array}{cc}
\ce{CH3}\\
|\\
\ce{CH3-C-Cl}\phantom{...}\\
|\\
\ce{CH3}\\
\ce{(III)}
\end{array}\]
What is dehydrohalogenation? State the rule for the formation of the preferred product of dehydrohalogenation.
The least reactive towards nucleophilic addition reactions is ____________.
Which of the following is least reactive towards SN1 reactions?
Dehydrohalogenation of an alkyl halide is ____________.
Nitroalkanes are obtained in laboratory from primary or secondary alkyl halides by the action of ______.
Which of the following carbocation is the most stable?
When C2H5Br is treated with excess of alc. NH3, the major product obtained is ____________.
The SN2 reaction of a compound containing an asymmetric carbon atom always gives ____________.
Identify 'B' in the following reaction.
\[\ce{2-Bromobutane ->[KOH alc.][\Delta] A ->[HI] B}\]
What is the major product obtained in the sulphonation of chlorobenzene with concentrated sulphuric acid?
The major product formed when 2-bromobutane is treated with alcoholic KOH is ______.
The compound that reacts the fastest with sodium methoxide is ______.
Complete the following reaction giving major products.
\[\begin{array}{cc}
\ce{CH3}\phantom{.......}\\
|\phantom{.........}\\
\ce{CH3 - c - CH2 - Cl ->[Na/dry ether]}\\
|\phantom{.........}\\
\ce{CH3}\phantom{.......}
\end{array}\]
Observe the following and answer the question given below:
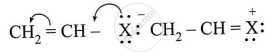
Comment on the bond length of C-X bond in it.
Observe the following and answer the question given below:
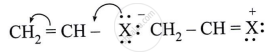
Can react by SN1 mechanism? Justify your answer.
Define and explain the SN1 mechanism with a suitable example.
What is the action of following on ethyl bromide?
alcoholic sodium hydroxide
Complete the reaction:
\[\ce{CH3CH2Cl ->[AgCN][alc.\Delta]}\] ?
Complete the following reaction giving major product.
\[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
|\phantom{...................}\\
\ce{CH3 - C - CH2 - Cl ->[Na/dry ether] A}\\
|\phantom{...................}\\
\ce{CH3}\phantom{.................}
\end{array}\]
Complete the following reaction giving major product.
\[\begin{array}{cc}
\ce{CH3}\phantom{................}\\
|\phantom{...................}\\
\ce{CH3 - C - CH2 - Cl ->[Na/dry ether] A}\\
|\phantom{...................}\\
\ce{CH3}\phantom{................}\\
\end{array}\]
