Advertisements
Advertisements
Question
Predict all the alkenes that would be formed by dehydrohalogenation of the following halide with sodium ethoxide in ethanol and identify the major alkene:
2-Chloro-2-methylbutane
Solution
2-Chloro-2-methylbutane has two different sets of equivalent β-hydrogens so that it will give 2 alkenes.
\[\begin{array}{cc}
\ce{\overset{β}{C}H3}\phantom{.................................}\ce{CH3}\phantom{..................}\ce{CH3}\phantom{..}\\
\phantom{}|\phantom{....................................}|\phantom{......................}|\phantom{....}\\
\ce{\overset{β}{C}H3 - C - \overset{β}{C}H2CH3 ->[C2H5ONa/C2H5OH][-HCl] \underset{2-Methylbut-1-ene}{CH2 = C - CH2CH3} + \underset{2-Methylbut-2-ene}{CH3 - C = CHCH3}}\\
|\phantom{...............................................................}\\
\ce{\underset{2-Chloro-2-methylbutane}{Cl}}\phantom{..............................................................}
\end{array}\]
Since the more substituted alkene will be more stable, 2-methylbut-2-ene will be the major alkene.
APPEARS IN
RELATED QUESTIONS
Identify the product ‘D’ in the following sequence of reactions:
\[\ce{H3C - CH2 - CH2 - Cl \underset{KOH}{\overset{Alc}{->}} 'B' \overset{HBr}{->} 'C' \underset{Elther}{\overset{Na}{->}}'D'}\]
Write the main products when n-butyl chloride is treated with alcoholic KOH.
Predict all the alkenes that would be formed by dehydrohalogenation of the following halide with sodium ethoxide in ethanol and identify the major alkene:
1-Bromo-1-methylcyclohexane
Predict all the alkenes that would be formed by dehydrohalogenation of the following halide with sodium ethoxide in ethanol and identify the major alkene:
2, 2, 3-Trimethyl-3-bromopentane
How will you bring about the following conversion?
But-1-ene to but-2-ene
How the following conversion can be carried out?
1-Bromopropane to 2-bromopropane
How the following conversion can be carried out?
2-Chloropropane to 1-propanol
How the following conversion can be carried out?
2-Bromopropane to 1-bromopropane
Observe the following compounds and answer the questions given below.
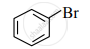
(I)
\[\ce{\underset{\text{(II)}}{CH3 - CH2 - Br}}\]
- Identify the type of halides.
- Explain the nature of the C – Br bond in both of these halides.
- Which of these compounds will undergo aqueous alkaline hydrolysis readily? Write the reaction in support of your answer.

'A' is:
Deamination of meso- di bromobutane gives mainly:-
Identify the major product formed when 2-cyclohexylchloroethane undergoes a dehydrohalogenation reaction. Name the reagent which is used to carry out the reaction.
The conversion of an alkyl halide into an alkene by alcoholic KOH is classified as ______.
Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
\[\ce{CH3 C(CI) (C2H5)CH2CH3 }\]
