Advertisements
Advertisements
Question
Predict all the alkenes that would be formed by dehydrohalogenation of the following halide with sodium ethoxide in ethanol and identify the major alkene:
1-Bromo-1-methylcyclohexane
Solution

In this compound, all β-hydrogen atoms are equal. Dehydrohalogenation of this chemical gives only one alkene.
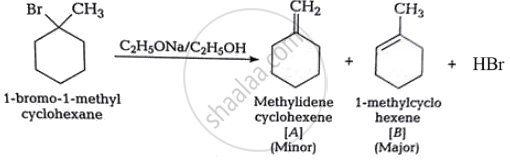
APPEARS IN
RELATED QUESTIONS
Identify the product ‘D’ in the following sequence of reactions:
\[\ce{H3C - CH2 - CH2 - Cl \underset{KOH}{\overset{Alc}{->}} 'B' \overset{HBr}{->} 'C' \underset{Elther}{\overset{Na}{->}}'D'}\]
Identify ‘A’ and ‘B’ in the following reaction :
\[\ce{CH3 - CH = CH2 ->[HBr]'A' ->[alc.KOH]'B'}\]
How do you convert: 2-bromobutane to but-2-ene
Write the main products when n-butyl chloride is treated with alcoholic KOH.
Predict all the alkenes that would be formed by dehydrohalogenation of the following halide with sodium ethoxide in ethanol and identify the major alkene:
2-Chloro-2-methylbutane
Predict all the alkenes that would be formed by dehydrohalogenation of the following halide with sodium ethoxide in ethanol and identify the major alkene:
2, 2, 3-Trimethyl-3-bromopentane
How will you bring about the following conversion?
But-1-ene to but-2-ene
How the following conversion can be carried out?
1-Bromopropane to 2-bromopropane
How the following conversion can be carried out?
2-Chloropropane to 1-propanol
Draw a neat, labelled energy profile diagram for SN1 reaction mechanism.
What are racemates?
Deamination of meso- di bromobutane gives mainly:-
Elimination of bromine from 2-bromobutane results in the formation of ______.
The conversion of an alkyl halide into an alkene by alcoholic KOH is classified as ______.
Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
\[\ce{CH3 C(CI) (C2H5)CH2CH3 }\]
