Advertisements
Advertisements
Question
How the following conversion can be carried out?
2-Chloropropane to 1-propanol
Solution
\[\begin{array}{cc}
\ce{CH3 - CH - CH3 ->[Alc. KOH, \Delta][Dehydrohalogenation] \underset{Propene}{CH3 - CH = CH2} ->[HBr,Peroxide] \underset{1-Bromopropane}{CH3 - CH2 - CH2 - Br} ->[KOH (aq.), \Delta][nucleophilic substitution] \underset{1-propanol}{CH3 - CH2 - CH2 - OH}}\\
|\phantom{.....................................................................................................................}\\
\ce{\underset{2-Chloropropane}{Cl}}\phantom{....................................................................................................................}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Identify the product ‘D’ in the following sequence of reactions:
\[\ce{H3C - CH2 - CH2 - Cl \underset{KOH}{\overset{Alc}{->}} 'B' \overset{HBr}{->} 'C' \underset{Elther}{\overset{Na}{->}}'D'}\]
State and explain Markownikoff's rule with suitable example
How do you convert: 2-bromobutane to but-2-ene
Predict all the alkenes that would be formed by dehydrohalogenation of the following halide with sodium ethoxide in ethanol and identify the major alkene:
1-Bromo-1-methylcyclohexane
Predict all the alkenes that would be formed by dehydrohalogenation of the following halide with sodium ethoxide in ethanol and identify the major alkene:
2-Chloro-2-methylbutane
Predict all the alkenes that would be formed by dehydrohalogenation of the following halide with sodium ethoxide in ethanol and identify the major alkene:
2, 2, 3-Trimethyl-3-bromopentane
How will you bring about the following conversion?
But-1-ene to but-2-ene
How the following conversion can be carried out?
1-Bromopropane to 2-bromopropane
How the following conversion can be carried out?
2-Bromopropane to 1-bromopropane
Draw a neat, labelled energy profile diagram for SN1 reaction mechanism.
What are racemates?
Observe the following compounds and answer the questions given below.
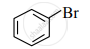
(I)
\[\ce{\underset{\text{(II)}}{CH3 - CH2 - Br}}\]
- Identify the type of halides.
- Explain the nature of the C – Br bond in both of these halides.
- Which of these compounds will undergo aqueous alkaline hydrolysis readily? Write the reaction in support of your answer.

'A' is:
Deamination of meso- di bromobutane gives mainly:-
Identify the major product formed when 2-cyclohexylchloroethane undergoes a dehydrohalogenation reaction. Name the reagent which is used to carry out the reaction.
The conversion of an alkyl halide into an alkene by alcoholic KOH is classified as ______.
Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
\[\ce{CH3 C(CI) (C2H5)CH2CH3 }\]
