Advertisements
Advertisements
Question
Stable form of A may be represented by the formula:
Options
A
A2
A3
A4
Solution
A
Explanation:
The electronic configuration of A is 1s2, 2s2, 2p6 having no unpaired electrons in the outermost shells. So, an atom having no unpaired electron in its valence shell is said to be stable.
APPEARS IN
RELATED QUESTIONS
Discuss the shape of the following molecules using the VSEPR model:
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
Select and write the most appropriate alternatives from the given choices.
Valence Shell Electron Pair repulsion (VSEPR) theory is used to predict which of the following:
Explain VSEPR theory. Applying this theory to predict the shapes of IF7 and SF6.
Identify the molecule with linear geometry?
Explain the non-linear shape of \[\ce{H2S}\] and non-planar shape of \[\ce{PCl3}\] using valence shell electron pair repulsion theory.
Which of the possible molecule/species is having maximum values for dipole moment. (where "A" is the central atom)?
Number of lone pair(s) of electrons on central atom and the shape of BrF3 molecule respectively are ______.
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: The H-O-H bond angle in water molecule is 104.5°.
Reason R: The lone pair-lone pair repulsion of electrons is higher than the bond pair-bond pair repulsion.
In the light of the above statements, choose the correct answer from the options given below:
In the following structure, the percentage of the 's' character in the lone pair occupy by the oxygen atom is ______.
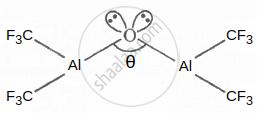
Given: Cos θ = −0.99
What is the geometry of a water molecule?
