Advertisements
Advertisements
Question
State the order of filling atomic orbitals following Aufbau principle.
Solution
Aufbau principle:
- The Aufbau principle gives the sequence in which various orbitals are filled with electrons.
- In the ground state of an atom, the orbitals are filled with electrons based on the increasing order of energies of orbitals, Pauli’s exclusion principle, and Hund’s rule of maximum multiplicity.
- Increasing order of energies of orbitals:
a. Orbitals are filled in order of increasing value of (n + 1)
b. In cases where the two orbitals have the same value of (n + 1), the orbital with a lower value of n is filled first. - The increasing order of energy of different orbitals in a multi-electron atom is:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s and so on.
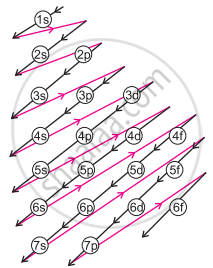
Increasing order of orbital energy
APPEARS IN
RELATED QUESTIONS
Using s, p, d notations, describe the orbital with the following quantum numbers n = 1, l = 0.
Using s, p, d notations, describe the orbital with the following quantum numbers n = 4; l =3.
Choose the correct option.
p-orbitals are _________ in shape.
Choose the correct option.
“No two electrons in the same atoms can have identical set of four quantum numbers”. This statement is known as -
Define the term Electronic configuration
Write orbital notations for the electron in orbitals with the following quantum numbers.
n = 2, l = 1
Write orbital notations for the electron in orbitals with the following quantum numbers.
n = 3, l = 2
Write electronic configurations of \[\ce{Fe, Fe2+, Fe3+}\].
Write condensed orbital notation of electronic configuration of the following element:
Carbon (Z = 6)
Write condensed orbital notation of electronic configuration of the following element:
Chlorine (Z = 17)
Draw shapes of 2s orbitals.
Explain in brief, the significance of the azimuthal quantum number.
The electronic configuration of oxygen is written as 1s2 2s2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^1_{{y}}}\] \[\ce{2p^1_{{z}}}\] and not as 1s2 2s2 \[\ce{2p^2_{{x}}}\], \[\ce{2p^2_{{y}}}\] \[\ce{2p^0_{{z}}}\], Explain.
Write a note on ‘Principal Quantum number.
Indicate the number of unpaired electron in:
Cr (Z = 24)
The principal quantum number (n) and magnetic quantum number (ml) for the valence electrons of rubidium atom (Z = 37) are ____________ respectively.
How many electrons can fit in the orbital for which n = 4 and l = 2?
Nickel atom can lose two electrons to form \[\ce{Ni^{2+}}\] ion. The atomic number of nickel is 28. From which orbital will nickel lose two electrons.
Which of the following orbitals are degenerate?
3dxy, 4dxy 3dz2, 3dyz, 4dyz, 4dz2
The arrangement of orbitals on the basis of energy is based upon their (n + l) value. Lower the value of (n + l), lower is the energy. For orbitals having same values of (n + l), the orbital with lower value of n will have lower energy.
Based upon the above information, arrange the following orbitals in the increasing order of energy.
1s, 2s, 3s, 2p
The arrangement of orbitals on the basis of energy is based upon their (n + l) value. Lower the value of (n + l), lower is the energy. For orbitals having same values of (n + l), the orbital with lower value of n will have lower energy.
Based upon the above information, arrange the following orbitals in the increasing order of energy.
4s, 3s, 3p, 4d
The arrangement of orbitals on the basis of energy is based upon their (n + l) value. Lower the value of (n + l), lower is the energy. For orbitals having same values of (n + l), the orbital with lower value of n will have lower energy.
Based upon the above information, solve the questions given below:
Which of the following orbitals has the lowest energy?
4d, 4f, 5s, 5p
The electronic configuration of valence shell of Cu is 3d104s1 and not 3d94s2. How is this configuration explained?
Match the following
| (i) Photon | (a) Value is 4 for N shell |
| (ii) Electron | (b) Probability density |
| (iii) ψ2 | (c) Always positive value |
| (iv) Principal quantum number n | (d) Exhibits both momentum and wavelength |
Choose the INCORRECT statement
Which of the following is the correct plot for the probability density ψ2 (r) as a function of distance 'r' of the electron from the nucleus for 2s orbitals?
Which one of the following laws will represent the pairing of electrons in a subshell after each orbital is filled with one electron?
