Advertisements
Advertisements
Question
The energy levels of an atom of a certain element are shown in the given figure. Which one of the transitions A, B, C, D or E will result in the emission of photons of electromagnetic radiation of wavelength 618.75 nm? Support your answer with mathematical calculations.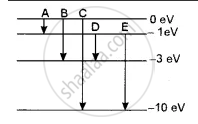
Solution
The wavelength emitted λ = 618.75 nm
∴ Corresponding energy E = h `c/λ`
= `( 6.6 xx 10^-34 xx 3 xx 10^8 )/( 618.75 xx 10^-9 xx 1.6 xx 10^-19) e^V`
= `( 6.6 xx 3 )/( 618.75 xx 1.6 ) xx 100`
= 2eV
This corresponds to the transition D.
In which energy emitted is ΔE = - 1 - ( - 3 ) = 2eV
APPEARS IN
RELATED QUESTIONS
Write any two important properties of electromagnetic waves.
An e.m. wave is propagating in a medium with a velocity `vec"v" = "v" hat"i"`. The instantaneous oscillating electric field of this e.m. wave is along +y-axis, then the direction of an oscillating magnetic field of the e.m. wave will be along:
Let E = E0 sin[106 x -ωt] be the electric field of plane electromagnetic wave, the value of ω is ______.
Explain the Maxwell’s modification of Ampere’s circuital law.
Discuss the source of electromagnetic waves.
Which of these mechanisms can be used to produce electromagnetic waves?
Why is the orientation of the portable radio with respect to broadcasting station important?
A plane EM wave travelling along z direction is described by `E = E_0 sin (kz - ωt)hati` and `B = B_0 sin (kz - ωt)hatj`. show that
- The average energy density of the wave is given by `u_(av) = 1/4 ε_0E_0^2 + 1/4 B_0^2/mu_0`.
- The time averaged intensity of the wave is given by `I_(av) = 1/2 cε_0 E_0^2`.
A plane electromagnetic wave of frequency 500 MHz is travelling in a vacuum along a y-direction.
At a particular point in space and time, `vec"B"` = 8.0 × 10-8 `hat"Z"`T. The value of the electric field at this point is ______.
(speed of light = 3 × 108 ms-1)
`hat x, hat y, hat z` are unit vectors along x, y, and Z directions.
Name the electromagnetic wave/radiation which is used to study crystal structure.
