Advertisements
Advertisements
Question
The outer surface of a cylinder containing a gas is rubbed vigorously by a polishing machine. The cylinder and its gas become warm. Is the energy transferred to the gas heat or work?
Solution
As the outer surface of a cylinder containing a gas is rubbed vigorously by a polishing machine, no work is done on the cylinder. Volume of the gas remains constant and the heat energy generated due to friction between the machine and the cylinder gets transferred to the gas as heat energy. This heat energy leads to an increase in the temperature of the cylinder and its gas.
APPEARS IN
RELATED QUESTIONS
In changing the state of a gas adiabatically from an equilibrium state A to another equilibrium state B, an amount of work equal to 22.3 J is done on the system. If the gas is taken from state A to B via a process in which the net heat absorbed by the system is 9.35 cal, how much is the net work done by the system in the latter case? (Take 1 cal = 4.19 J)
Two cylinders A and B of equal capacity are connected to each other via a stopcock. A contains a gas at standard temperature and pressure. B is completely evacuated. The entire system is thermally insulated. The stopcock is suddenly opened. Answer the following:
Do the intermediate states of the system (before settling to the final equilibrium state) lie on its P-V-T surface?
A steam engine delivers 5.4×108 J of work per minute and services 3.6 × 109 J of heat per minute from its boiler. What is the efficiency of the engine? How much heat is wasted per minute?
Should the internal energy of a system necessarily increase if heat is added to it?
When we rub our hands they become warm. Have we supplied heat to the hands?
Can work be done by a system without changing its volume?
In a process on a system, the initial pressure and volume are equal to the final pressure and volume.
(a) The initial temperature must be equal to the final temperature.
(b) The initial internal energy must be equal to the final internal energy.
(c) The net heat given to the system in the process must be zero.
(d) The net work done by the system in the process must be zero.
A 100 kg lock is started with a speed of 2.0 m s−1 on a long, rough belt kept fixed in a horizontal position. The coefficient of kinetic friction between the block and the belt is 0.20. (a) Calculate the change in the internal energy of the block-belt system as the block comes to a stop on the belt. (b) Consider the situation from a frame of reference moving at 2.0 m s−1 along the initial velocity of the block. As seen from this frame, the block is gently put on a moving belt and in due time the block starts moving with the belt at 2.0 m s−1. calculate the increase in the kinetic energy of the block as it stops slipping past the belt. (c) Find the work done in this frame by the external force holding the belt.
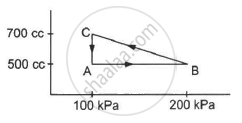
A gas is taken through a cyclic process ABCA as shown in figure. If 2.4 cal of heat is given in the process, what is the value of J ?
A mixture of fuel and oxygen is burned in a constant-volume chamber surrounded by a water bath. It was noticed that the temperature of water is increased during the process. Treating the mixture of fuel and oxygen as the system,
- Has heat been transferred?
- Has work been done?
- What is the sign of ∆U?
Define heat.
What is the internal energy of the system, when the amount of heat Q is added to the system and the system does not do any work during the process?
When does a system lose energy to its surroundings and its internal energy decreases?
One gram of water (1 cm3) becomes 1671 cm3 of steam at a pressure of 1 atm. The latent heat of vaporization at this pressure is 2256 J/g. Calculate the external work and the increase in internal energy.
A cylinder containing one gram molecule of the gas was compressed adiabatically until its temperature rose from 27°C to 97°C. Calculate the work done and heat produced in the gas (𝛾 = 1.5).
In a thermodynamic system, working substance is ideal gas. Its internal energy is in the form of ______.
8 m3 of a gas is heated at the pressure 105 N/m2 until its volume increases by 10%. Then, the external work done by the gas is ____________.
Two samples A and B, of a gas at the same initial temperature and pressure are compressed from volume V to V/2; A isothermally and B adiabatically. The final pressure of A will be ______.
Which of the following represents isothermal process?
Explain the change in internal energy of a thermodynamic system (the gas) by heating it.
