Advertisements
Advertisements
Question
What are the different types of thermodynamic systems?
Solution
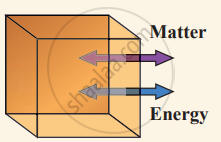
(i) Open system can exchange both matter and energy with the environment.
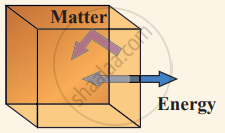
(ii) Closed system exchange energy but not matter with the environment.
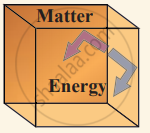
(iii) Isolated system can exchange neither energy nor matter with the environment.
APPEARS IN
RELATED QUESTIONS
A gas contained in a cylinder fitted with a frictionless piston expands against a constant external pressure of 1 atm from a volume of 5 liters to a volume of 10 liters. In doing so it absorbs 400J of thermal energy from its surroundings. Determine the change in the internal energy of the system.
A hypothetical thermodynamic cycle is shown in the figure. Calculate the work done in 25 cycles.

A group of objects that can form a unit which may have the ability to exchange energy with its surrounding is called what?
A hot cup of coffee is kept on the table. After some time it attains a thermal equilibrium with the surroundings. By considering the air molecules in the room as a thermodynamic system, which of the following is true
What is meant by ‘thermal equilibrium’?
What are intensive and extensive variables? Give examples.
Discuss the thermal equilibrium.
Discuss the mechanical equilibrium.
Which of the following statements is correct for any thermodynamic system?
What are surroundings in thermodynamics?
