Advertisements
Advertisements
Question
What is meant by the following term? Give an example of the reaction in the following case.
2, 4-DNP-derivative
Solution
When an aldehyde or ketone reacts with 2, 4-dinitrophenylhydrazine in a weak acidic medium, 2, 4-dinitrophenylhydrazone (2, 4-DNP derivative) is produced.
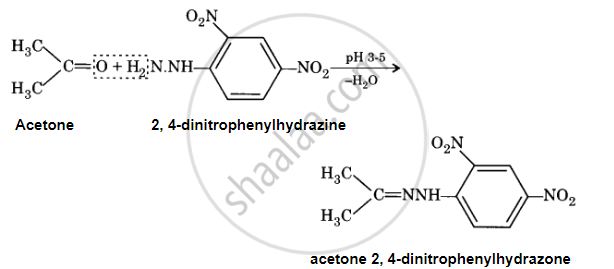
APPEARS IN
RELATED QUESTIONS
Acetaldehyde, when treated with which among the following reagents does NOT undergo addition reaction?
(A) Ammonia
(B) Hydroxylamine
(C) Ammoniacal silver nitrate
(D) Semicarbazide
Write balanced chemical equations for action of ammonia on - formaldehyde
Predict the products of the following reactions:
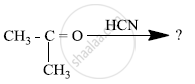
Write the products formed when CH3CHO reacts with the following reagents : H2N – OH
Predict the product of the following reaction:
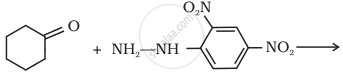
Predict the product of the following reaction:
\[\begin{array}{cc}
\phantom{..............}\ce{O}\\
\phantom{..............}||\\
\ce{R - CH = CH - CHO + NH2 - C - NH - NH2 ->[H+]}\end{array}\]
What is meant by the following term? Give an example of the reaction in the following case.
Acetal
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.
PhMgBr and then H3O+
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.
Excess ethanol and acid
Give plausible explanation for the following:
There are two −NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
How will you convert benzoic acid to m-bromobenzoic acid?
Alkenes 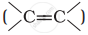 and carbonyl compounds
and carbonyl compounds 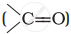 , both contain a π bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
, both contain a π bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
Carboxylic acids contain carbonyl group but do not show the nucleophilic addition reaction like aldehydes or ketones. Why?
Grignard reagent on reaction with acetone forms.
What happens when ethanal is treated with excess ethanol and acid?
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
Draw the structure of the following derivative.
Acetaldehydedimethylacetal
Draw structure of the following derivative:
Acetaldehydedimethylacetal
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
Draw structures of the given derivatives.
The ethylene ketal of hexan-3-one
