Advertisements
Advertisements
Question
When phenolphthalein is added to NaOH, the colour of the solution will become _________.
- colourless
- red
- pink
- yellow
Solution
pink
When phenolphthalein is added to NaOH, the colour of the solution will become pink
APPEARS IN
RELATED QUESTIONS
10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralise it will be:
Name one natural source of the following acids:
Lactic acid
Complete and balance the following chemical equations
Na2 CO3 (s) + HCI (aq) →
Complete and balance the following chemical equations:
NaOH (aq) HCI (aq)→
Compounds such as alcohol and glucose also contain hydrogen but are not categorised as acids. Describe an activity to prove it.
A solution reacts with marble chips to produce a gas which turns lime water milky. The solution contains:
(a) Na2SO4
(b) CaSO4
(c) H2SO4
(d) K2SO4
A substance X which is used as an antacid reacts with dilute hydrochloric acid to produce a gas Y which is used in one type of fire-extinguisher. Name the substance X and gas Y. Write a balanced equation for the chemical reaction which takes place.
What happens when a solution of sodium hydrogencarbonate is heated? Write equation of the reaction involved.
What does a soda-acid type fire extinguisher contain? How does it work? Explain the working of a soda-acid fire extinguisher with the help of a labelled diagram.
If you take some distilled water in a test-tube, add an equal amount of acetic acid to it, shake the test-tube well and leave it undisturbed on the test-tube stand, then after about 5 minutes what would you observe?
(A) There is a layer of water over the layer of acetic acid.
(B) A precipitate is setting at the bottom of the test-tube.
(C) Bubbles of colourless gas are coming out of the test-tube.
(D) There is a clear, colourless transparent solutions in the test-tube.
Read the following statements:
I. When a red litmus paper is dipped into reaction mixture of a saponification reaction, it turns blue and the reaction is exothermic.
II. When a blue litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is exothermic.
III. When a red litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is endothermic.
IV. When a blue litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is endothermic.
Which of the above statements are correct:
(A) I, and II
(B) II and III
(C) III and IV
(D) I and IV
When _______________ is passed through fresh lime water, it turns milky.
Magnesium hydroxides are used for treating _______
Egg shell is made up of ____________.
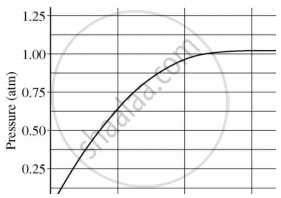
A student added 10 g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition take place?
A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue?
Which of the following statements is not correct?
Acids present in fruits and vegetables are called ______ acids.
Vinegar is ______ in taste.
