Advertisements
Advertisements
Question
Which among the following statements is incorrect for magnesium metal?
Options
It burns in oxygen with a dazzling white flame
It reacts with cold water to form magnesium oxide and evolves hydrogen gas
It reacts with hot water to form magnesium hydroxide and evolves hydrogen gas
It reacts with steam to form magnesium hydroxide and evolves hydrogen gas
Solution
It reacts with cold water to form magnesium oxide and evolves hydrogen gas
Explanation -
Magnesium does not react with cold water. It only reacts with hot water or steam to form magnesium hydroxide and hydrogen gas.
APPEARS IN
RELATED QUESTIONS
How will you obtain Zinc chloride from zinc.
Also give balanced equations for the reactions
What is meant by the metal reactivity series ? State its importance, (any two points).
Write chemical equation for the event.
Iron filings are dropped in aqueous solution of copper sulphate.
Divide the metals Cu, Zn, Ca, Mg, Fe, Na, Li into three groups, namely reactive metals, moderately reactive metals and less reactive metals.
With reference to Acid explain with a suitable example of how the reactivity of the metals could be differentiated.
Explain the following reaction with the balanced equation.
Sodium burns in air
Explain the following reaction with the balanced equation.
Sulphur burns in air
Observe the following diagram and identify the type of reaction and write observation.
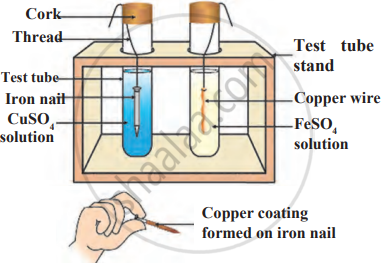
A metal M does not liberate hydrogen from acids but reacts with oxygen to give a black colour product. Identify M and black coloured product and also explain the reaction of M with oxygen.
Arrange the following as per the instruction given in the bracket:
Al, K, Mg, Ca (decreasing order of its reactivity)
