Advertisements
Advertisements
Question
Explain the following reaction with the balanced equation.
Sulphur burns in air
Solution
Sulphur burns in air to form sulphur dioxide.
\[\ce{\underset{\text{Sulphur}}{S} + \underset{\text{Oxygen}}{O2} -> \underset{\text{Sulphur dioxide}}{SO2}}\]
APPEARS IN
RELATED QUESTIONS
What do you observe when dilute sulphuric acid is added to granulated zinc?
What do you observe when ferrous sulphate solution is added to an aqueous solution of sodium hydroxide.
Fill in the blank
When a piece of copper is added to silver nitrate solution, it turns ............in colour.
Write chemical equation for the event.
Iron filings are dropped in aqueous solution of copper sulphate.
Write chemical equation for the event.
Electrolysis of alumina is done.
Divide the metals Cu, Zn, Ca, Mg, Fe, Na, Li into three groups, namely reactive metals, moderately reactive metals and less reactive metals.
State what is meant by the ‘reactivity series of metals’
With reference to Water explain with suitable examples of how the reactivity of the metals could be differentiated.
With reference to Acid explain with a suitable example of how the reactivity of the metals could be differentiated.
Give a balanced equation for the reversible catalytic reaction involving nitrogen as one of the reactants.
Select the correct answer for the statement given below:
The catalyst used in the catalytic reaction involving the reactants nitrogen and hydrogen.
Select the correct answer for the statement given below:
A neutral oxide which does not react with an acid or a base to give salt and water.
Complete the statement by filling in the blank with the correct word:
The metal which reacts with steam and the reaction is reversible is ________.
In preparation of Aqua regia hydrochloric acid and _______ acid are mixed.
Classify the following metals based on their reactivity.
Cu, Zn, Ca, Mg, Fe, Na, Li, Hg
| More reactive | Moderately reactive | Less reactive |
Explain the following reaction with the balanced equation.
Magnesium reacts with dil HCl
Observe the following diagram and identify the type of reaction and write observation.
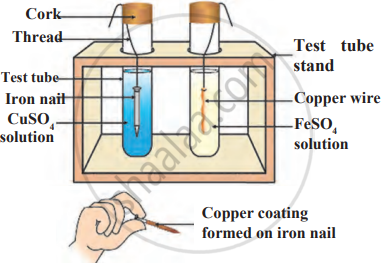
Which among the following statements is incorrect for magnesium metal?
Compound X and aluminium are used to join railway tracks.
- Identify the compound X
- Name the reaction
- Write down its reaction.
Of the three metals X, Y and Z. X reacts with cold water, Y with hot water and Z with steam only. Identify X, Y and Z and also arrange them in order of increasing reactivity.
