Advertisements
Advertisements
Question
Write chemical equation for the event.
Electrolysis of alumina is done.
Solution 1
The electrolysis of alumina is carried out in a steel tank lined inside with graphite. The graphite lining serves as cathode. Anode is also made up of graphite rods hanging in the molten mass. The electrolyte consists of alumina dissolved in fused Cryolite(Na3AlF6) and Fluorspar(CaF2). Cryolite lowers the melting point of alumina and fluorspar increases the fluidity of the mass so that the liberated aluminum metal may sink at the bottom of the cell. When electric current is passed through this mixture, the aluminum is collected at the cathode in molten state and sinks at the bottom.

Ionization of Alumina:2Al2O3 → 6O-2 + 4Al+3
Reaction at Cathode: 4Al+3 + 12e- → 4Al
Reaction at Anode: 6O-2 → 3O2 + 12e-, C + O2 → CO2
Solution 2
When electrolysis of alumina is done, aluminium is formed at the cathode and oxygen gas liberated at the anode.
Cathode:
\[\ce{Al^{3+} + 3e^- -> Al_{(l)}}\] (Reduction)
Anode:
\[\ce{2O^2- -> O_{2(g)} + 4e^-}\] (Oxidation)
RELATED QUESTIONS
What do you observe when dilute sulphuric acid is added to granulated zinc?
What do you observe when a few pieces of iron are dropped in a blue solution of copper sulphate?
How will you obtain Silver chloride from silver nitrate.
Also give balanced equations for the reactions
How will you obtain Zinc chloride from zinc.
Also give balanced equations for the reactions
What do you observe when Magnesium ribbon is burnt in oxygen.
What is meant by the metal reactivity series ? State its importance, (any two points).
Write chemical equation for the event.
Iron filings are dropped in aqueous solution of copper sulphate.
Write the chemical equation for the event.
A reaction was brought about between ferric oxide and aluminum.
Write a chemical equation for the following event.
Zinc oxide is dissolved in dilute hydrochloric acid.
With reference to Acid explain with a suitable example of how the reactivity of the metals could be differentiated.
In preparation of Aqua regia hydrochloric acid and _______ acid are mixed.
Explain the following reaction with the balanced equation.
Sodium burns in air
Observe the following diagram and identify the type of reaction and write observation.
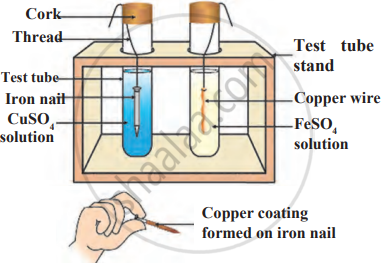
Which among the following alloys contain mercury as one of its constituents?
A metal that exists as a liquid at room temperature is obtained by heating its sulphide in the presence of air. Identify the metal and its ore and give the reaction involved.
Of the three metals X, Y and Z. X reacts with cold water, Y with hot water and Z with steam only. Identify X, Y and Z and also arrange them in order of increasing reactivity.
An element A burns with golden flame in air. It reacts with another element B, atomic number 17 to give a product C. An aqueous solution of product C on electrolysis gives a compound D and liberates hydrogen. Identify A, B, C and D. Also write down the equations for the reactions involved.
Arrange the following as per the instruction given in the bracket:
Al, K, Mg, Ca (decreasing order of its reactivity)
