Advertisements
Advertisements
Question
Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
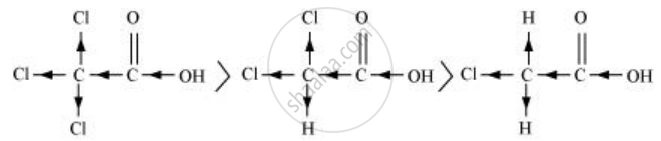
\[\ce{Cl3CCOOH > Cl2CHCOOH > ClCH2COOH}\]
Short Note
Solution
\[\ce{Cl3CCOOH > Cl2CHCOOH > ClCH2COOH}\]
The order of acidity can be explained on the basis of Inductive effect (–I effect). As the number of chlorine atoms increases, the –I effect increases. With the increase in –I effect, the acid strength also increases accordingly.
shaalaa.com
Fundamental Concepts in Organic Reaction Mechanism - Electron Displacement Effects in Covalent Bonds
Is there an error in this question or solution?
Chapter 12: Organic Chemistry - Some Basic Principles and Techniques - EXERCISES [Page 371]
