Advertisements
Advertisements
Questions
With the help of a circuit diagram describe the experiment to study the characteristics of the photoelectric effect. Hence discuss any 2 characteristics of the photoelectric effect.
With a neatly labelled circuit diagram, describe an experiment to study the characteristics of the photoelectric effect.
Solution
- A laboratory experimental set-up for the photoelectric effect consists of an evacuated glass tube with a quartz window.
- The glass tube contains photosensitive metal plates. One is the emitter E and another plate is the collector C.

Schematic of experimental set-up for the photoelectric effect - The emitter and collector are connected to a voltage source whose voltage can be changed and to an ammeter to measure the current in the circuit.
- A potential difference of V, as measured by the voltmeter, is maintained between the emitter E and collector C. Generally, C (the anode) is at a positive potential with respect to the emitter E (the cathode). This potential difference can be varied and C can even be at a negative potential with respect to E.
- When the anode potential (V) is positive, it accelerates the electrons. This potential is called accelerating potential. When the anode potential (V) is negative, it retards the flow of electrons. This potential is known as retarding potential.
- A source S of monochromatic light of sufficiently high frequency (short wavelength ≤ 10–7 m) is used.
Two characteristics of the photoelectric effect:
- The photoelectric work function `phi_0` is constant for a given emitter. Hence if the frequency ‘ν’ of the incident radiation is decreased, the maximum kinetic energy of the emitted photoelectrons decreases, till it becomes zero for a certain frequency ν0.
Therefore, from Einstein’s equation,
0 = `"hv"_0 - phi_0`
∴ `phi_0 = "hv"_0` ........(1)
This shows that the threshold frequency is related to the work function of the metal and hence it has different values for different metals. - The photoelectric equation is,
`1/2"mv"_"max"^2 = "hv" - phi_0` ........(2)
where, hν = energy of the photon of incident radiation.
`phi_0 = "hv"_0` = photoelectric work function of the metal.
Thus, both the terms on the R.H.S of equation (2) depend on the frequency and not on the intensity of radiation. Hence the maximum kinetic energy with which photoelectrons are emitted is independent of the intensity of radiation. However, since `phi_0` and h are constants, the maximum kinetic energy of the photoelectrons is directly proportional to the frequency.
RELATED QUESTIONS
If the frequency of incident light falling on a photosensitive material is doubled, then the kinetic energy of the emitted photoelectron will be ______.
What is the photoelectric effect?
Using the values of work function given in the following table, tell which metal will require the highest frequency of incident radiation to generate photocurrent.
Typical values of work function for some common metals
| Metal | Work function (in eV) |
| Potassium | 2.3 |
| Sodium | 2.4 |
| Calcium | 2.9 |
| Zinc | 3.6 |
| Silver | 4.3 |
| Aluminium | 4.3 |
| Tungsten | 4.5 |
| Copper | 4.7 |
| Nickel | 5.0 |
| Gold | 5.1 |
It is observed in an experiment on the photoelectric effect that an increase in the intensity of the incident radiation does not change the maximum kinetic energy of the electrons. Where does the extra energy of the incident radiation go? Is it lost? State your answer with explanatory reasoning.
The electrons are emitted in the photoelectric effect from a metal surface.
As the intensity of incident light increases ______
The maximum kinetic energy of the photoelectrons depends only on ______
The maximum velocity of the photoelectron emitted by the metal surface is 'v '. Charge and mass of the photoelectron is denoted by 'e' and 'm' respectively. The stopping potential in volt is ______.
Threshold frequency for a metal is 1015 Hz. Light of `lambda` = 4000 Å falls on its surface. Which of the following statements is correct?
In photoelectric experiment, if both the intensity and frequency of the incident light are doubled, then the saturation of photoelectric current ______.
An important spectral emission line has a wavelength of 21 cm. The corresponding photon energy is (h = 6.62 x 10-34 Js, c = 3 x 108 m/s) ____________.
A metal surface is illuminated by photons of energy 5 eV and 2.5 eV respectively. The ratio of their wavelengths is ____________.
In photoelectric effect, graph of saturation current versus frequency of light is plotted. The nature of the graph will be ____________.
Light of frequency 2 times the threshold frequency is incident on a photo sensitive material. If the frequency is made `1/3`rd and intensity is doubled then the photocurrent will ______.
When wavelength of incident radiation on the metal surface is reduced from 'λ1' to 'λ2', the kinetic energy of emitted photoelectrons is tripled. The work function of the metal is ______.
(h = Planck's constant, c =velocity of light)
When light of wavelength 'λ' is incident on a photosensitive surface, the stopping potential is 'V'. When light of wavelength '3λ' is incident on the same surface, the stopping potential is `"V"/6`. Threshold wavelength for the surface is _______.
Photoelectrons are emitted from a photosensitive surface for the light of wavelengths λ1 = 360 nm and λ2 = 600 nm. What is the ratio of work functions for lights of wavelength 'λ1' to 'λ2'?
When a photosensitive surface is irradiated by lights of wavelengths `lambda_1` and `lambda_2`, kinetic energies of emitted photoelectrons are E1 and E2 respectively. The work function of the photosensitive surface is ____________.
Two incident radiations having energies two times and ten times of the work function of a metal surface, produce photoelectric effect. The ratio of maximum velocities of emitted photo electrons respectively is ____________.
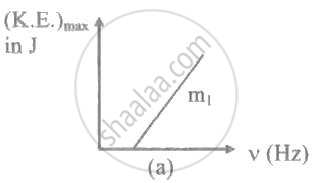
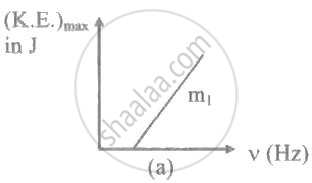
The ratio of slopes m1: ro2 of the lines given in the following graphs is, ______.
Which one of the following graphs represents the variation of photoelectric current (i) with intensity (I) of the incident light?
In a photoelectric experiment, ultraviolet light of wavelength 280 nm is used with a lithium cathode having work function Φ = 2.5 eV. If the wavelength of incident light is switched to 400 nm, find out the change in the stopping potential.
(h = 6.63 × 10-34 Js, c = 3 × 108 ms-1)
When radiation of wavelength λ is used to illuminate a metallic surface, the stopping potential is V. When the same surface is illuminated with radiation of wavelength 3λ, the stopping potential is `"V"/4`. If the threshold wavelength for the metallic surface is nλ. then value of n will be ______.
The radiation emitted, when an electron jumps from n = 3 to n = 2 orbit is a hydrogen atom, falls on a metal to produce photoelectron. The electrons from the metal surface with maximum kinetic energy are made to move perpendicular to a magnetic field of `1/320`T in a radius of 10-3m. Find the 320 work function of metal:
The wavelength of light incident on a metal surface is reduced from 300 nm to 200 nm (both are less than threshold wavelength). What is the change in the stopping potential for photoelectrons emitted from the surface will be ______ V. (Take h = 6.6 × 10-34 J-s)
For a given photosensitive material and frequency (> threshold frequency) of incident radiation, the photoelectric current varies with the intensity of incident light as:
On a photosensitive material when frequency of incident radiation is increased by 30%, kinetic energy of emitted photoelectrons increases from 0.4 eV. The work function of the surface is ______.
The photoelectric threshold for a certain metal surface is 3600 Å. If the metal surface is irradiated by a wavelength of 1100 Å, then kinetic energy of the emitted photoelectrons is ______.
