Advertisements
Advertisements
Question
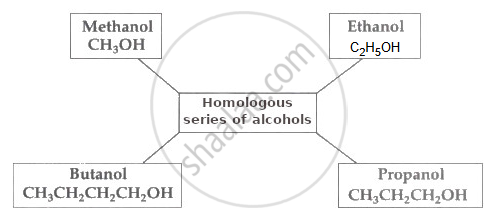
Write names of first four homologous series of alcohols.
Solution
Name of first four homologous series of alcohols.
RELATED QUESTIONS
Write the name and formula of the 2nd member of homologous series having general formula CnH2n + 2.
Write the molecular formula of first two members of homologous series having functional group – OH
Fill in the following blank with suitable word:
Ethene and ethyne are examples of ..... hydrocarbons.
What is the difference between two consecutive homologues:
(1) in terms of molecular mass?
(2) in terms of number and kind of atoms per molecule?
The molecular formula of an organic compound is C18H36. Name its homologous series.
A colourless organic liquid X of molecular formula C2H4O2 turns blue litmus to red. Another colourless organic liquid Y of molecular formula C3H6O has no action on any litmus but it is used as a nail polish remover. A yet another colourless organic liquid Z of molecular formula C2H6O has also no action on litmus but it is used in tincture of iodine.
(a) Name the liquid X. To which homologous series does it belong? Give the name of another member of this homologous series.
(b) Name the liquid Y. To which homologous series does it belong? Write the name of another member of this homologous series.
(c) Can you name an organic compound having the same molecular formula as liquid Y but which belongs to a different homologous series? What is this homologous series?
(d) Name the liquid Z. To which homologous series does it belong? Write the name of another member of this homologous series.
What is homologous series ?
What is the difference in the molecular formula of any two adjacent homologues in terms of molecular mass?
Why homologous series of carbon compounds are so called? Write chemical formula of two consecutive members of a homologous series and state the part of these compounds that determines their (i) physical properties, and (ii) chemical properties.
Copy and complete the following table which relates to three homologus series of hydrocarbons:
| General formula | CnH2n | CnH2n-2 | CnH2n+2 |
| IUPAC name of the homologus series | |||
| Characteristic bond type | Single bonds | ||
| IUPAC name of the first member of the series | |||
| Type of reaction with chlorine | Addition |
What is the difference in the molecular formula of any two adjacent homologues:
(i) In terms of molecular mass
(ii) In terms of number and kind of atoms per molecule?
The phenomenon in which compounds having different structural formulae have the same molecular formula is called _______.
While going in an increasing order there is a rise in the molecular mass of the consecutive members of the homologous series by _______.
Complete the following table for the homologous series of alkanes.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Methane | CH4 | CH4 | 1 | 1 | -162 |
| Ethane | C2H6 | CH3–CH3 | 2 | 2 | -88.5 |
| Propane | C3H8 | CH3–CH2–CH3 | 3 | 3 | -42 |
| Butane | C4H10 | CH3–CH2–CH2–CH3 | ______ | ______ | 0 |
| Pentane | C5H12 | CH3–CH2–CH2–CH2–CH3 | ______ | ______ | 36 |
| Hexane | C6H14 | CH3–CH2–CH2–CH2–CH2–CH3 | ______ | ______ | 69 |
Which of the following does not belong to the same homologous series?
Consider the carbon compounds having following molecular formula:
(i) C3H6 (ii) C3H8 (iii) C4H6 (iv) C6H6 (v) C6H12
- State the number of double covalent bonds present in C3H6.
- Write the formula of first member of the homologous series to which the carbon compound C4H6 belongs.
- Which one of the above compounds forms a ring structure of carbon atoms?
- Identify, which of the above compounds, is a member of alkane series.
Name and draw the electron dot structure of first homologue of alkynes series.
Name the following:
Group of organic compounds where the successive members follow a regular structural pattern, successive compounds differ by a 'CH2' group.
