Advertisements
Advertisements
Question
Write nuclear reaction equation for β+-decay of `""_6^11"C"`.
Solution
α is a nucleus of helium `(""_2^4"He")` and β is an electron (e− for β− and e+ for β+). In every α-decay, there is a loss of 2 protons and 4 neutrons. In every β+-decay, there is a loss of 1 proton and a neutrino is emitted from the nucleus. In every β−-decay, there is a gain of 1 proton and an antineutrino is emitted from the nucleus.
For the given case, the various nuclear reaction can be written as:
`""_6^11"C" -> _5^11"B" + "e"^+ + "v"`
APPEARS IN
RELATED QUESTIONS
The half life of a certain radioactive material against \u0003α-decay is 100 days. After how much time, will the undecayed fraction of the material be 6.25%?
For the past some time, Aarti had been observing some erratic body movement, unsteadiness and lack of coordination in the activities of her sister Radha, who also used to complain of severe headache occasionally. Aarti suggested to her parents to get a medical check-up of Radha. The doctor thoroughly examined Radha and diagnosed that she has a brain tumour.
(a) What, according to you, are the values displayed by Aarti?
(b) How can radioisotopes help a doctor to diagnose brain tumour?
Draw graphs showing variation of photoelectric current with applied voltage for two incident radiations of equal frequency and different intensities. Mark the graph for the radiation of higher intensity.
Define ‘activity’ of a radioactive material and write its S.I. units.
The sequence of stepwise decay of a radioactive nucleus is
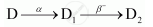
If the atomic number and mass number of D2 are 71 and 176 respectively, what are their corresponding values of D?
State the law of radioactive decay. hence derive the relation N = Noe-λt . Represent it graphically.
The half-life of 199Au is 2.7 days. (a) Find the activity of a sample containing 1.00 µg of 198Au. (b) What will be the activity after 7 days? Take the atomic weight of 198Au to be 198 g mol−1.
Radioactive 131I has a half-life of 8.0 days. A sample containing 131I has activity 20 µCi at t = 0. (a) What is its activity at t = 4 days? (b) What is its decay constant at t = 4.0 days?
The count rate from a radioactive sample falls from 4.0 × 106 per second to 1.0 × 106per second in 20 hours. What will be the count rate 100 hours after the beginning?
The half-life of 226Ra is 1602 y. Calculate the activity of 0.1 g of RaCl2 in which all the radium is in the form of 226Ra. Taken atomic weight of Ra to be 226 g mol−1 and that of Cl to be 35.5 g mol−1.
`""_80^197`Hg decay to `""_79^197`Au through electron capture with a decay constant of 0.257 per day. (a) What other particle or particles are emitted in the decay? (b) Assume that the electron is captured from the K shell. Use Moseley's law √v = a(Z − b) with a = 4.95 × 107s−1/2 and b = 1 to find the wavelength of the Kα X-ray emitted following the electron capture.
4 × 1023 tritium atoms are contained in a vessel. The half-life of decay tritium nuclei is 12.3 y. Find (a) the activity of the sample, (b) the number of decay in the next 10 hours (c) the number of decays in the next 6.15 y.
238U decays to 206Pb with a half-life of 4.47 × 109 y. This happens in a number of steps. Can you justify a single half for this chain of processes? A sample of rock is found to contain 2.00 mg of 238U and 0.600 mg of 206Pb. Assuming that all the lead has come from uranium, find the life of the rock.
Radioactive isotopes are produced in a nuclear physics experiment at a constant rate dN/dt = R. An inductor of inductance 100 mH, a resistor of resistance 100 Ω and a battery are connected to form a series circuit. The circuit is switched on at the instant the production of radioactive isotope starts. It is found that i/N remains constant in time where i is the current in the circuit at time t and N is the number of active nuclei at time t. Find the half-life of the isotope.
`""_83^212"Bi"` can disintegrate either by emitting an α-particle of by emitting a β−-particle. (a) Write the two equations showing the products of the decays. (b) The probabilities of disintegration α-and β-decays are in the ratio 7/13. The overall half-life of 212Bi is one hour. If 1 g of pure 212Bi is taken at 12.00 noon, what will be the composition of this sample at 1 P.m. the same day?
In a gamma ray emission from nucleus :
Half-life of a certain radioactive material is 8 hours.
Find the disintegration constant of this material.
