Advertisements
Advertisements
Question
The sequence of stepwise decay of a radioactive nucleus is
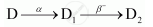
If the atomic number and mass number of D2 are 71 and 176 respectively, what are their corresponding values of D?
Solution
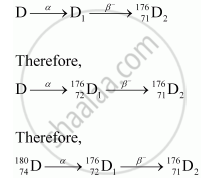
So, the corresponding values of atomic number and mass number for D are 74 and 180.
APPEARS IN
RELATED QUESTIONS
The half life of a certain radioactive material against \u0003α-decay is 100 days. After how much time, will the undecayed fraction of the material be 6.25%?
For the past some time, Aarti had been observing some erratic body movement, unsteadiness and lack of coordination in the activities of her sister Radha, who also used to complain of severe headache occasionally. Aarti suggested to her parents to get a medical check-up of Radha. The doctor thoroughly examined Radha and diagnosed that she has a brain tumour.
(a) What, according to you, are the values displayed by Aarti?
(b) How can radioisotopes help a doctor to diagnose brain tumour?
Write nuclear reaction equation for β+-decay of `""_6^11"C"`.
Write nuclear reaction equation for electron capture of `""_54^120"Xe"`.
Define ‘activity’ of a radioactive material and write its S.I. units.
A certain sample of a radioactive material decays at the rate of 500 per second at a certain time. The count rate falls to 200 per second after 50 minutes. (a) What is the decay constant of the sample? (b) What is its half-life?
A vessel of volume 125 cm3 contains tritium (3H, t1/2 = 12.3 y) at 500 kPa and 300 K. Calculate the activity of the gas.
Plot a graph showing the variation of undecayed nuclei N versus time t. From the graph, find out how one can determine the half-life and average life of the radioactive nuclei.
Complete the following nuclear reactions :
(i) `"_15^32P -> ` `"_z^AX + bar(e) + bar(v)`
(ii) `"_6^12 C `+`"_6^12C ->` ` "_2^A Y + ` `"_4^2 He`
The half-life of a certain radioactive element is 3.465 days. Find its disintegration constant.
