ISC (Commerce)
ISC (Arts)
ISC (Science)
Academic Year: 2018-2019
Date: March 2019
Advertisements
Fill in the blanks by choosing the appropriate word/words from those given in the brackets:
(more than, primary, cathode, Lucas reagent, two, four, less than, Grignard’s reagent, tertiary anode, zero, equal to, three)
The elevation of boiling point of 0.5 M K2SO4 solution is ________ that of 0.5 M urea solution. The elevation of boiling point of 0.5 M KCl solution is _______ that of 0·5 M K2SO4 solution.
Chapter: [0.013000000000000001] Relative molecular mass of non-volatile substances
Fill in the blanks by choosing the appropriate word/words from those given in the brackets:
(more than, primary, cathode, Lucas reagent, two, four, less than, Grignard’s reagent, tertiary anode, zero, equal to, three)
A mixture of conc. HCl and anhydrous ZnCl2 is called _______ which shows maximum reactivity with _________ alcohol.
Chapter: [0.0302] Collision Theory [0.0304] Effect of temperature on the rate constant of a reaction
Fill in the blanks by choosing the appropriate word/words from those given in the brackets:
In electrolytic refining the impure metal is made ________ while a thin sheet of pure metal is used as ___________.
Chapter: [0.012] Dissociation- Electrolytic solute
Fill in the blanks by choosing the appropriate word/words from those given in the brackets:
When the concentration of reactant of first order reaction is doubled, the rate of reaction becomes ________ times, but for a ________ order reaction, the rate of reaction remains the same.
Chapter: [0.030699999999999998] Meaning of Chemical Kinetics
Select the correct alternative from the choices given :
The cell reaction is spontaneous or feasible when emf of the cell is:
negative
positive
zero
either positive or negative
Chapter: [0.065] Batteries
Select the correct alternative from the choices given :
Which, among the following polymers, is a polyester:
melamine
bakelite
terylene
polythene
Chapter: [0.16] Polymers
Select the correct alternative from the choices given :
The correct order of increasing acidic strength of the oxoacids of chlorine is:
HClO3 < HClO4 < HClO2 < HClO
HClO < HClO2 < HClO3 < HClO4
HClO2 < HClO < HClO4 < HClO3
HClO3 < HClO4 < HClO < HClO2
Chapter: [0.08] Chemistry of p-Block Elements: Group 16, 17, 18
Select the correct alternative from the choices given :
A catalyst is a substance which:
changes the equilibrium constant of the reaction
increases the equilibrium constant of the reaction
supplies energy to the reaction
shortens the time reach equilibrium
Chapter: [0.030699999999999998] Meaning of Chemical Kinetics
Match the following:
(i) Diazotisation (a) Anisotropic
(ii) Crystalline solid (b) Reimer-Tiemann reaction
(iii) Phenol (c) Diphenyl
Chapter: [0.030699999999999998] Meaning of Chemical Kinetics
Answer the followiiig questions:
Which trivalent ion has maximum size in the Lanthanoid series i.e. Lanthanum ion (La3+) to Luteium ion (Lu3+)?
(at. no. of Lanthanum = 57 and Lutetium = 71)
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Answer the followiiig questions:
When coordination compound 3 3 .6 CoCl NH is mixed with 3, AgNO three moles of AgCl are precipitated per mole of the compound. Write the stuctural formula and IUPAC name of the coordination compound.
[ Co ( NH3)6 ] Cl3
Hexamine cobalt (III) chloride
Chapter: [0.07] Coordination Compounds
Answer the followiiig questions:
Calculate the boiling point of urea solution when 6 g of urea is dissolved in 200 g of water.
(Kb for water = 0.52 K kg mol–1, bolining point of pure water = 373 K, mol. wt. of urea = 60)
Chapter: [0.013000000000000001] Relative molecular mass of non-volatile substances
Answer the followiiig questions:
Identify the compounds A, B, C and D in the given reaction:
Chapter: [0.131] Carbonyl compounds
For a reaction , A + B → C+D the initial rate for different reactions and initial concentrations of reactants are given below:
| S.NO. | Initial Conc. | Initial rate (mole L–1 sec–1) | |
| [ A] mole L–1 | [B] mole L–1 | ||
| 1 | 1.0 | 1.0 | `2xx10^-3` |
| 2 | 2.0 | 1.0 | `4xx10^-3` |
| 3 | 4.0 | 1.0 | `8xx10^-3` |
| 4 | 1.0 | 2.0 | `2xx10^-3` |
| 5 | 1.0 | 4.0 | `2xx10^-3` |
(i) What is the overall order of reaction?
(ii) Write the rate law equation.
Chapter: [0.030699999999999998] Meaning of Chemical Kinetics
25% of first order reaction is completed in 30 minutes. Calculate the time taken in minutes for the reaction to go to 90% completion.
Chapter: [0.030699999999999998] Meaning of Chemical Kinetics
Name the type of drug which lowers the body temperature in high fever condition.
Chapter: [0.18] Chemistry in Everyday Life
What are tranquilizers? Give one example of tranquilizer.
Chapter: [0.18] Chemistry in Everyday Life
Advertisements
Write the balanced chemical equation of the following:
Chlorobenzene treated with ammonia in the presence of Cu2O at 475 K and 60 atm.
Chapter: [0.114] Chlorobenzene
Write the balanced chemical equation of the following:
Ethyl chloride treated with alcoholic potassium hydroxide.
Chapter: [0.115] Preparation, properties, and uses of the following
Name the monomer and the type of polymerisation that takes place when PTFE is formed.
Chapter: [0.16] Polymers
Name the monomers of nylon 6, 6.
Chapter: [0.16] Polymers
Name two water-soluble vitamins and the diseases caused by their deficiency in the diet of an individual.
Chapter: [0.17] Biomolecules – carbohydrates, proteins, enzymes, vitamins and nucleic acids
How will you obtain the following? (Give balanced chemical equation):
Benzene from phenol.
Chapter: [0.114] Chlorobenzene
How will you obtain the following? (Give balanced chemical equation):
Iodoform from ethanol
Chapter: [0.114] Chlorobenzene
Give a balanced equation for the preparation of salicylaldehyde from phenol.
Chapter: [0.121] Methods of preparation, manufacture, properties and use
How will you obtain the following? (Give balanced chemical equation): Propan-2-ol from Grignard’s reagent.
Chapter: [0.122] Preparation, properties and uses of ethane-1, 2 diol, propane-1, 2, 3 triol
Show that for a first-order reaction the time required to complete 75% of reaction is about 2 times more than that required to complete 50% of the reaction.
Chapter: [0.0301] Order of a reaction
When 0.4g of oxalic acid is dissolved in 40g of benzene, the freezing point of the solution is lowered by 0.45 K. Calculate the degree of association of acetic acid. Acetic acid forms dimer when dissolved in benzene.
(Kf for benzene = 5.12 K kg mol-1, at. wt. C = 12, H = 1, O = 16)
Chapter: [0.142] Acid derivatives
A solution is prepared by dissolving 9.25 g of non-volatile solute in 450 mL of water. It has an osmotic pressure of 350 mm of Hg at 27°C. Assuming the solute is non-electrolyte, determine its molecular mass. (R = 0.0821 lit atm K-1 mol-1)
Chapter: [0.011000000000000001] Raoult's law and colligative properties
An element occurs in a body-centred cubic structure. Its density is 8.0 g/cm3. If the cell edge is 250 pm, calculate the atomic mass of an atom of this element. (N0 = 6.023 × 1023)
Chapter: [0.02] States of Matters: Structure and Properties Solid State
Describe the role of the Cryolite in the extraction of aluminium from pure alumina.
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Describe the role of the NaCN in the extraction of silver from silver ore.
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
What is the role of coke in the extraction of iron from its oxides?
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Write the IUPAC name of the K3[Fe(C2O4)3].
Chapter: [0.07] Coordination Compounds
Write the IUPAC names of the Co(NH3)5ClSO4
Chapter: [0.07] Coordination Compounds
[Fe(CN)6]4- is a coordination complex ion.
Calculate the oxidation number of iron in the complex.
Chapter: [0.057] Common ion effect
[Fe(CN)6]4- is a coordination complex ion.
Is the complex ion diamagnetic or paramagnetic?
Chapter: [0.057] Common ion effect
[Fe(CN)6]4- is a coordination complex ion.
What is the hybridisation state of the central metal atom?
Chapter: [0.057] Common ion effect
[Fe(CN)6]4- is a coordination complex ion.
Write the IUPAC name of the complex ion.
Chapter: [0.057] Common ion effect
Explain why transition elements form alloys.
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Explain why Zn2+ salts are white whereas Cu2+ salts are coloured.
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Explain why transition metals and their compounds act as a catalyst.
Chapter: [0.1] Chemistry of Transition and Inner-Transition Elements: d-Block: 3d, 4d and 5d series f-Block: 4f and 5f series
Complete and balance the chemical equations:
KMnO4 + H2SO4 + H2C2O4 → ____ + ____ + ____ + _____
Chapter: [0.115] Preparation, properties, and uses of the following
Complete and balance the chemical equation:
\[\ce{K2Cr2O7 + H2SO4 + KI →}\] _____ + _____ + _____ + _____.
Chapter: [0.115] Preparation, properties, and uses of the following
Complete and balance the chemical equations:
K2Cr2O7 + H2SO4 + FeSO4 → _____ + _____ + _____ + _____
Chapter: [0.115] Preparation, properties, and uses of the following
Advertisements
Give balanced equation for the following:
Aniline is treated with bromine water.
Chapter: [0.15] Cyanides, Isocyanides, Nitro compounds, Amines and Diazonium Salt
Give balanced equation for the following:
Ethylamine is heated with chloroform and alcoholic solution of potassium hydroxide.
Chapter: [0.115] Preparation, properties, and uses of the following
Give balanced equation for the following:
Benzene diazonium chloride is treated with an ice-cold solution of aniline in acidic medium.
Chapter: [0.115] Preparation, properties, and uses of the following
Define the following term with suitable examples:
Peptisation
Chapter: [0.012] Dissociation- Electrolytic solute
Define the following term with suitable examples:
Electrophoresis.
Chapter: [0.012] Dissociation- Electrolytic solute
Define the following term with suitable examples:
Dialysis
Chapter: [0.013000000000000001] Relative molecular mass of non-volatile substances
Calculate the mass of silver deposited at cathode when a current of 2 amperes is passed through a solution of AgNO3 for 15 minutes.
(at. wt. of Ag= 108, 1F = 96500 C)
Chapter: [0.02] States of Matters: Structure and Properties Solid State
Calculate the emf and ΔG for the cell reaction at 298 K
Mg(s)|Mg2+(0.1 M) || CU2+(0.01 M)|Cu(s)
Given E°cell = 2.71 V
1F = 96,500 C
Chapter: [0.02] States of Matters: Structure and Properties Solid State
Define the following term Specific conductance.
Chapter: [0.064] Electrolytic conductance
Define the following term Kohlrausch’s Law.
Chapter: [0.064] Electrolytic conductance
Explain why:
(1) Fluorine has a lower electron affinity than chlorine.
(2) Red phosphorus is less reactive than white phosphorus.
(3) Ozone acts as a powerful oxidising agent.
Chapter: [0.08] Chemistry of p-Block Elements: Group 16, 17, 18 [0.09] Preparation/ Manufacture, Properties and Uses of Compounds of Groups 16, 17, – Ozone, Sulphur Dioxide, Sulphuric Acid, Hydrochloric Acid
Draw the structures of the following :
(1) XeF6
(2) IF7
Chapter: [0.07] Coordination Compounds
Explain why:
(1) Interhalogen compounds are more reactive than the related elemental halogens.
(2) Sulphur exhibits the tendency for catenation but oxygen does not.
(3) On being slowly passed through water, PH3 forms bubbles but NH3 dissolves.
Chapter: [0.08] Chemistry of p-Block Elements: Group 16, 17, 18
Complete and balance the following reactions:
(1) P4 + H2SO4 → ____ + _____ + _____ + _____
(2) Ag + HNO3(dilute) → _____ + ______ + _____ + _____
Chapter: [0.07] Coordination Compounds
Give balanced chemical equations for the following reactions:
(1) Acetaldehyde reacts with hydrogen cyanide.
(2) Acetone reacts with phenylhydrazine.
(3) Acetic acid is treated with ethanol and a drop of conc. H2SO4.
Chapter: [0.131] Carbonyl compounds
Give one chemical test each to distinguish between the following pairs of compounds:
(1) Acetone and benzaldehyde.
(2) Phenol and benzoic acid.
Chapter: [0.131] Carbonyl compounds
Write a chemical equation to illustrate the following name reaction:
Aldol condensation.
Chapter: [0.131] Carbonyl compounds
Write a chemical equation to illustrate the following name reaction:
Cannizzaro’s reaction.
Chapter: [0.131] Carbonyl compounds
Write a chemical equation to illustrate the following name reaction:
Benzoin condensation
Chapter: [0.131] Carbonyl compounds
Identify the compounds A and B in the given reactions:
(1)
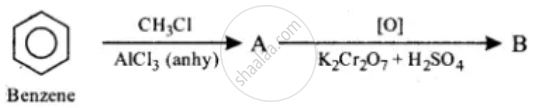
(2)
\[\ce{CH3COCH3 ->[{[O]}][HNO3(conc.)]A->[PCl5]B}\]
Chapter: [0.131] Carbonyl compounds
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CISCE previous year question papers Class 12 Chemistry (Theory) with solutions 2018 - 2019
Previous year Question paper for CISCE Class 12 -2019 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry (Theory), you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CISCE Class 12.
How CISCE Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry (Theory) will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
