Advertisements
Advertisements
Question
Draw the structures of the following :
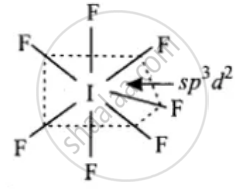
(1) XeF6
(2) IF7
Solution
(1)
XeF6 has distorted octahedral structure

(2)
IF7 has the pentagonal bipyramidal structure
APPEARS IN
RELATED QUESTIONS
The hexaquo manganese (II) ion contains five unpaired electrons, while the hexacyanoion contains only one unpaired electron. Explain using Crystal Field Theory.
Why are low spin tetrahedral complexes rarely observed?
Write the electronic configuration of Fe(III) on the basis of crystal field theory when it forms an octahedral complex in the presence of (i) strong field, and (ii) weak field ligand. (Atomic no.of Fe=26)
The colour of the coordination compounds depends on the crystal field splitting. What will be the correct order of absorption of wavelength of light in the visible region, for the complexes, \[\ce{[Co(NH3)6]^{3+}}\], \[\ce{[Co(CN)6]^{3-}}\], \[\ce{[Co(H2O)6]^{3+}}\]
The CFSE for octahedral \[\ce{[CoCl6]^{4-}}\] is 18,000 cm–1. The CFSE for tetrahedral \[\ce{[CoCl4]^{2-}}\] will be ______.
Atomic number of \[\ce{Mn}\], \[\ce{Fe}\] and \[\ce{Co}\] are 25, 26 and 27 respectively. Which of the following inner orbital octahedral complex ions are diamagnetic?
(i) \[\ce{[Co(NH3)6]^{3+}}\]
(ii) \[\ce{[Mn(CN)6]^{3-}}\]
(iii) \[\ce{[Fe(CN)6]^{4-}}\]
(iv) \[\ce{[Fe(CN)6]^{3-}}\]
On the basis of crystal field theory explain why Co(III) forms paramagnetic octahedral complex with weak field ligands whereas it forms diamagnetic octahedral complex with strong field ligands.
Why are low spin tetrahedral complexes not formed?
Give the electronic configuration of the following complexes on the basis of Crystal Field Splitting theory.
\[\ce{[CoF6]^{3-}, [Fe(CN)6]^{4-} and [Cu(NH3)6]^{2+}}\].
The complex that has highest crystal field splitting energy (Δ) is ______.
