Advertisements
Advertisements
Define terms: Fuel cell
Concept: Batteries > Primary Batteries
A first-order reaction is 50% completed in 40 minutes at 300 K and in 20 minutes at 320 K. Calculate the activation energy of the reaction. (Given : log 2 = 0·3010, log 4 = 0·6021, R = 8·314 JK–1 mol–1)
Concept: Temperature Dependence of the Rate of a Reaction
Give reason for the following:
Primary amines have higher boiling point than tertiary amines.
Concept: Physical Properties of Amines
On the basis of crystal field theory, write the electronic configuration for d4 ion if ∆0 < P.
Concept: Bonding in Coordination Compounds > Crystal Field Theory (CFT)
State Henry’s law.
Concept: Solubility > Solubility of a Gas in a Liquid
Define azeotropes.
Concept: Vapour Pressure of Liquid > Vapour Pressure of Liquid- Liquid Solutions
Account for the following :
Zn is not considered as a transition element.
Concept: General Properties of the Transition Elements (D-block)
Write the chemical equations to illustrate the following name reactions : Rosenmund reduction
Concept: Aldehydes and Ketones > Preparation of Aldehydes
What is meant by positive deviations from Raoult's law? Give an example. What is the sign of ∆mixH for positive deviation?
Concept: Vapour Pressure of Liquid > Vapour Pressure of Liquid- Liquid Solutions
What is ferromagnetism?
Concept: Magnetic Properties
An element crystallizes in a f.c.c. lattice with a cell edge of 250 pm. Calculate the density if 300 g of this element contains 2 × 1024 atoms.
Concept: Calculations Involving Unit Cell Dimensions
An element with density 2.8 g cm–3 forms a f.c.c. unit cell with edge length 4 x 10–8 cm. Calculate the molar mass of the element.
(Given : NA = 6.022 x 1023 mol –1)
Concept: Calculations Involving Unit Cell Dimensions
The rate constant for the first-order decomposition of H2O2 is given by the following equation:
`logk=14.2-(1.0xx10^4)/TK`
Calculate Ea for this reaction and rate constant k if its half-life period be 200 minutes.
(Given: R = 8.314 JK–1 mol–1)
Concept: Temperature Dependence of the Rate of a Reaction
From the given cells:
Lead storage cell, Mercury cell, Fuel cell and Dry cell
Answer the following:
(i) Which cell is used in hearing aids?
(ii) Which cell was used in Apollo Space Programme?
(iii) Which cell is used in automobiles and inverters?
(iv) Which cell does not have long life?
Concept: Batteries > Primary Batteries
Write the IUPAC name of the given compound:
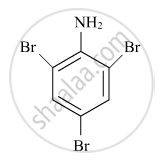
Concept: Nomenclature of Coordination Compounds - Naming of Mononuclear Coordination Compounds
When a co-ordination compound CrCl3.6H2O is mixed with AgNO3, 2 moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex.
(ii) IUPAC name of the complex.
Concept: Nomenclature of Coordination Compounds - Naming of Mononuclear Coordination Compounds
Write down the IUPAC name of the following complex: [Cr(NH3)2Cl2(en)]Cl (en = ethylenediamine)
Concept: Nomenclature of Coordination Compounds - Naming of Mononuclear Coordination Compounds
Draw the geometrical isomers of complex [Pt(NH3)2Cl2].
Concept: Isomerism in Coordination Compounds > Stereoisomerism
Write the IUPAC name of the complex [Cr(NH3)4 Cl2]Cl.
Concept: Nomenclature of Coordination Compounds - Naming of Mononuclear Coordination Compounds
Using IUPAC norms write the formulae for Potassium trioxalatoaluminate(III)
Concept: Importance and Applications of Coordination Compounds
