Advertisements
Advertisements
प्रश्न
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[FeF6]3−
उत्तर
In this complex, the oxidation state of Fe is +3.
Orbitals of Fe+3 ion:
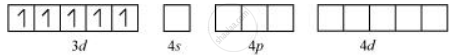
There are 6 F− ions. Thus, it will undergo d2sp3 or sp3d2 hybridization. As F− is a weak field ligand, it does not cause the pairing of the electrons in the 3d orbital. Hence, the most feasible hybridization is sp3d2.
sp3d2 hybridized orbitals of Fe are:
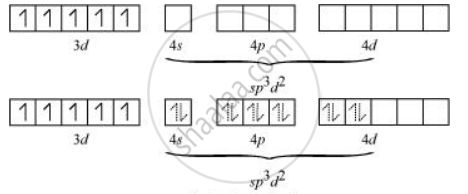
Hence, the geometry of the complex is found to be octahedral.
APPEARS IN
संबंधित प्रश्न
On the basis of valence bond theory explain the nature of bonding in [CoF6]3 ion.
Explain on the basis of valence bond theory that [Ni(CN)4]2− ion with square planar structure is diamagnetic and the [NiCl4]2− ion with tetrahedral geometry is paramagnetic.
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2− is diamagnetic. Explain why?
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[CoF6]3−
Write the hybridisation and number of unpaired electrons in the complex `[CoF_6]^(3-)`. (Atomic No. of Co = 27)
[NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why? (Atomic no. Ni = 28)
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Mn(CN)6]^{3-}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[FeCl6]^{4-}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
Write the hybridization and shape of the following complexes:
[Ni(CN)4]2−
In a coordination entity, the electronic configuration of the central metal ion is t2g3 eg1
Is the coordination compound a high spin or low spin complex?
When the hybridization state of carbon changes from sp3 to sp2 and finally to sp, the angle between hybridized orbital will
As the s-character of hybridised orbital increases, the bond angle
Which of the following methods is used for measuring bond length?
In Fe(CO)5, the Fe – C bond possesses
Using valence bond theory, predict the hybridization and magnetic character of the following:
[CoF6]3– [Atomic number of Co = 27]
According to the valence bond theory, the hybridization of central metal atom is dsp2 for which one of the following compounds?
The magnetic moment of [NiCl4]2− is ______.
[Atomic number: Ni = 28]
[Ni(CO)4] has tetrahedral geometry while [Ni(CN)4]2− has square planar, yet both exhibit diamagnetism. Explain.
[Atomic number: Ni = 28]
