Advertisements
Advertisements
प्रश्न
For M2+/M and M3+/M2+ systems, the EΘ values for some metals are as follows:
| Cr2+/Cr | −0.9 V |
| Mn2+/Mn | −1.2 V |
| Fe2+/Fe | −0.4 V |
| Cr3/Cr2+ | −0.4 V |
| Mn3+/Mn2+ | +1.5 V |
| Fe3+/Fe2+ | +0.8 V |
Use this data to comment upon:
The stability of Fe3+ in acid solution as compared to that of Cr3+ or Mn3+.
उत्तर
The value of EΘ for Cr3+/Cr2+ is negative. Hence Cr3+ is stable and cannot be reduced to Cr2+.
The value of EΘ for Mn3+/Mn2+ is more positive; hence, Mn3+ is not very stable and can easily be reduced to Mn2+. The value of EΘ for Fe3+/Fe2+ is less positive but smaller. Hence, Fe3+ is more stable than Mn3+, but it is less stable than Cr2+.
APPEARS IN
संबंधित प्रश्न
Out of Mn3+ and Cr3+, which is more paramagnetic and why ?
(Atomic nos. : Mn = 25, Cr = 24)
Describe the oxidising action of potassium dichromate and write the ionic equation for its reaction with iodide.
Predict which of the following will be coloured in the aqueous solution?
Ti3+, V3+, Cu+, Sc3+, Mn2+, Fe3+ and Co2+. Give reasons for each.
Write the formula of an oxo-anion of Chromium (Cr) in which it shows the oxidation state equal to its group number
Complete and balance the following chemical equations
`MnO_4^(-) + H_2O + I^(-) ->`
In lake test for Al3+ ions, there is the formation of coloured ‘floating lake’. It is due to ______.
Read the passage given below and answer the following question:
The transition metals when exposed to oxygen at low and intermediate temperatures form thin, protective oxide films of up to some thousands of Angstroms in thickness. Transition metal oxides lie between the extremes of ionic and covalent binary compounds formed by elements from the left or right side of the periodic table. They range from metallic to semiconducting and deviate by both large and small degrees from stoichiometry. Since electron bonding levels are involved, the cations exist in various valence states and hence give rise to a large number of oxides. The crystal structures are often classified by considering a cubic or hexagonal close-packed lattice of one set of ions with the other set of ions filling the octahedral or tetrahedral interstices. The actual oxide structures, however, generally show departures from such regular arrays due in part to distortions caused by packing of ions of different size and to ligand field effects. These distortions depend not only on the number of d-electrons but also on the valence and the position of the transition metal in a period or group.
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
Assertion: Crystal structure of oxides of transition metals often show defects.
Reason: Ligand field effect cause distortions in crystal structures.
The magnetic nature of elements depends on the presence of unpaired electrons. Identify the configuration of transition element, which shows highest magnetic moment.
Which of the following statements is not correct?
The halides of transition elements become more covalent with increasing oxidation state of the metal. Why?
Match the solutions given in Column I and the colours given in Column II.
| Column I (Aqueous solution of salt) |
Column II (Colour) |
| (i) \[\ce{FeSO2.7H2O}\] | (a) Green |
| (ii) \[\ce{NiCl2.4H2O}\] | (b) Light pink |
| (iii) \[\ce{MnCl2.4H2O}\] | (c) Blue |
| (iv) \[\ce{CoC12,6H2O}\] | (d) Pale green |
| (v) \[\ce{Cu2 Cl2}\] | (e) Pink |
| (f) Colourless |
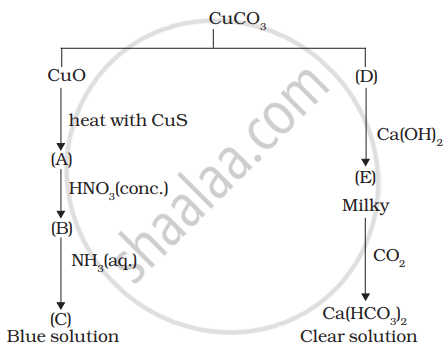
Identify A to E and also explain the reactions involved.
Mention any three processes where transition metals act as catalysts.
If enthalpies of formation of C2H4(g), CO2(g) and H2O(l) at 25°C and 1 atm pressure are 52, – 394 and – 286 kJ/mol respectively, the change in ethalpy for combustion of C2H4 is equal to
Which does not belong to first transition series?
The product of oxidation of I– with \[\ce{MnO^{-}4}\] in alkaline medium is:-
Which of the following ions has the maximum magnetic moment?
Which of the following transition metals shows +1 and +2 oxidation states?
Account for the following:
Copper has an exceptionally positive `"E"_("M"^(2+)//"M")^0` value.
The compounds of \[\ce{Ti^4+}\] ions are colourless due to ______.
