Advertisements
Advertisements
प्रश्न
Give a plausible explanation for the following:
Why are amines less acidic than alcohols of comparable molecular masses?
उत्तर
Loss of a proton from an amine gives an amide ion, while loss of a proton from alcohol gives an alkoxide ion.
\[\ce{R-NH2 -> R-NH– + H+}\]
\[\ce{R-O-H -> R-O^- + H+}\]
Since O is more electronegative than N, it will attract positive species more strongly in comparison to N. Thus, RO− is more stable than RNH−. Thus, alcohols are more acidic than amines. Conversely, amines are less acidic than alcohols.
APPEARS IN
संबंधित प्रश्न
Give the structure of A, B and C in the following reaction:
\[\ce{C6H5N2Cl ->[CuCN] A ->[H2O/H+] B ->[NH3][\Delta] C}\]
Give the structures of A, B and C in the following reactions :
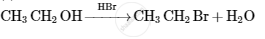
Account for the following:
Gabriel phthalimide synthesis is not preferred for preparing aromatic primary amines.
Write the order of reactivity of alkyl halides with ammonia.
Write reactions to bring about the following conversions.
Acetamide to Ethylamine
What product is formed when \[\ce{R - C ≡ N}\] is hydrolysed?
Identify the product obtained when benzamide is treated with bromine and aqueous sodium hydroxide?
The reduction of alkyl cyanide with sodium and ethanol to give primary amines is, ____________.
Which of the following compounds is obtained when quaternary ammonium hydroxide is strongly heated?
For producing amines, the reaction of nitro compounds with iron scrap is preferred because:
Which of the following compounds is the weakest Brönsted base?
Which of the following reactions are correct?
(i)
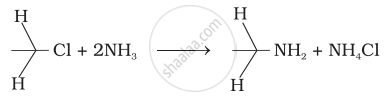
(ii)
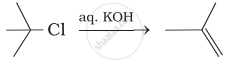
(iii)
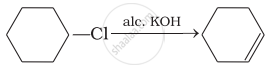
(iv)
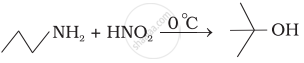
Write following conversions:
acetanilide `->` p-nitroaniline
Match the reactions given in Column I with the statements given in Column II.
| Column I | Column II | ||
| (i) | Ammonolysis | (a) | Amine with lesser number of carbon atoms |
| (ii) | Gabriel phthalimide synthesis | (b) | Detection test for primary amines. |
| (iii) | Hoffmann Bromamide reaction | (c) | Reaction of phthalimide with \[\ce{KOH}\] and \[\ce{R-X}\] |
| (iv) | Carbylamine reaction | (d) | Reaction of alkylhalides with \[\ce{NH3}\] |
The Gabriels' phthalimide synthesis is used in the synthesis of
Reduction of nitro alkanes yields which compound?
Give reasons for the following:
Ammonolysis of alkyl halides is not a good method to prepare pure primary amines.
Methyl amine on reaction with chloroform in the presence of NaOH gives ______.
Write short note on the following:
Ammonolysis
Write a short note on Ammonolysis.
