Advertisements
Advertisements
Question
Give a plausible explanation for the following:
Why are amines less acidic than alcohols of comparable molecular masses?
Solution
Loss of a proton from an amine gives an amide ion, while loss of a proton from alcohol gives an alkoxide ion.
\[\ce{R-NH2 -> R-NH– + H+}\]
\[\ce{R-O-H -> R-O^- + H+}\]
Since O is more electronegative than N, it will attract positive species more strongly in comparison to N. Thus, RO− is more stable than RNH−. Thus, alcohols are more acidic than amines. Conversely, amines are less acidic than alcohols.
APPEARS IN
RELATED QUESTIONS
Identify the compounds 'A' and 'B' in the following equation:

An aromatic compound 'A' of molecular formula C7H7ON undergoes a series of reactions as shown below. Write the structures of A, B, C, D and E in the following reactions :

Give the structures of A, B and C in the following reaction:
\[\ce{CH3COOH ->[NH3][\Delta] A ->[NaOBr] B ->[NaNO2/HCl] C}\]
Give the structures of A, B and C in the following reactions :
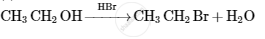
Choose the most correct option.
Which of the following compounds will dissolve in aqueous NaOH after undergoing reaction with Hinsberg reagent?
Why cannot aniline be prepared by Gabriel phthalimide synthesis?
Alkyl cyanides on reduction by sodium and ethanol give primary amines. This reaction is called as ____________.
Which of the following amines exhibits maximum degree of intermolecular hydrogen bonding?
What is the molar mass of the amine formed when acetamide undergoes Hofmann bromamide degradation?
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion (A): Alkyl halides are insoluble in water.
Reason (R): Alkyl halides have halogen attached to sp3 hybrid carbon.
Select the most appropriate answer from the options given below:
In order to prepare a 1° amine from an alkyl halide with simultaneous addition of one \[\ce{CH2}\] group in the carbon chain, the reagent used as source of nitrogen is ______.
Best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is ______.
Among the following amines, the strongest Brönsted base is:
Which of the following reactions are correct?
(i)
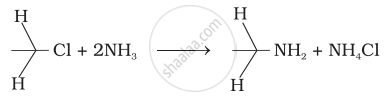
(ii)
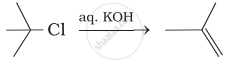
(iii)
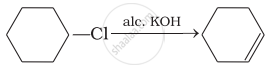
(iv)
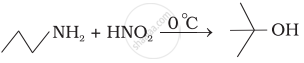
Match the reactions given in Column I with the statements given in Column II.
| Column I | Column II | ||
| (i) | Ammonolysis | (a) | Amine with lesser number of carbon atoms |
| (ii) | Gabriel phthalimide synthesis | (b) | Detection test for primary amines. |
| (iii) | Hoffmann Bromamide reaction | (c) | Reaction of phthalimide with \[\ce{KOH}\] and \[\ce{R-X}\] |
| (iv) | Carbylamine reaction | (d) | Reaction of alkylhalides with \[\ce{NH3}\] |
Assertion: Only a small amount of \[\ce{HCl}\] is required in the reduction of nitro compounds with iron scrap and \[\ce{HCl}\] in the presence of steam.
Reason: \[\ce{FeCl2}\] formed gets hydrolysed to release \[\ce{HCl}\] during the reaction.
In the given reaction what is the X?
\[\begin{array}{cc}
\ce{O}\phantom{.......................}\\
||\phantom{.......................}\\
\phantom{}\ce{R - C - OH <-[H3O] Χ ->[H] RCH2NH2}
\end{array}\]
What is the IUPAC name of \[\ce{(CH3)2 - N - CH3}\]?
Write short note on the following:
Ammonolysis
Assertion: Amimonolysis of alkyl halides involves the reaction between alkyl halides and alcoholic ammonia.
Reason: Ammonolysis of alkyl halides produces secondary amines only.
