Advertisements
Advertisements
प्रश्न
Give a plausible explanation for the following:
Why are amines less acidic than alcohols of comparable molecular masses?
उत्तर
Loss of a proton from an amine gives an amide ion, while loss of a proton from alcohol gives an alkoxide ion.
\[\ce{R-NH2 -> R-NH– + H+}\]
\[\ce{R-O-H -> R-O^- + H+}\]
Since O is more electronegative than N, it will attract positive species more strongly in comparison to N. Thus, RO− is more stable than RNH−. Thus, alcohols are more acidic than amines. Conversely, amines are less acidic than alcohols.
APPEARS IN
संबंधित प्रश्न
Write a short note on the following:
Hoffmann’s bromamide reaction
Give the structures of A, B and C in the following reaction:
\[\ce{C6H5NO2 ->[Fe/HCl] A ->[HNO2][273 K] B ->[C6H5OH] C}\]
Mention 'two' uses of propan-2-one.
Identify 'A' and 'B' in the following reaction and rewrite the complete reaction :
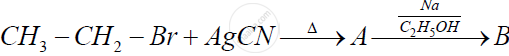
Give the structures of A, B and C in the following reactions :

What product is formed when \[\ce{R - C ≡ N}\] is hydrolysed?
The source of nitrogen in Gabriel synthesis of amines is ______.
Reduction of aromatic nitro compounds using \[\ce{Fe}\] and \[\ce{HCl}\] gives ______.
Reduction of nitrobenzene by which of the following reagent gives aniline?
(i) \[\ce{Sn/HCl}\]
(ii) \[\ce{Fe/HCl}\]
(iii) \[\ce{H2 - Pd}\]
(iv) \[\ce{Sn/NH4OH}\]
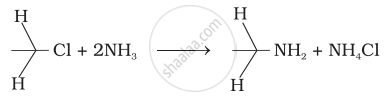
Which of the following reactions are correct?
(i)
(ii)
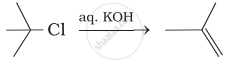
(iii)
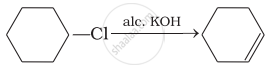
(iv)
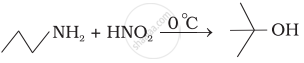
Identify A and B in the following reaction.
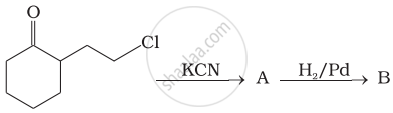
Write following conversions:
acetanilide `->` p-nitroaniline
How will you bring out the following conversion?

How will you carry out the following conversion?
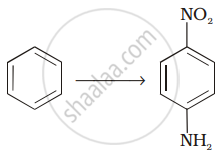
Assertion: Only a small amount of \[\ce{HCl}\] is required in the reduction of nitro compounds with iron scrap and \[\ce{HCl}\] in the presence of steam.
Reason: \[\ce{FeCl2}\] formed gets hydrolysed to release \[\ce{HCl}\] during the reaction.
A primary amine is formed by an amide on treatment with bromine and alkali. The primary amine has
When primary amines are treated with HCl, the product obtained is which of the following?
Which of the following compound gives pink colour on reaction with phthalic anhydride in cone. H2SO4 followed by treatment with NaOH?
- Phenyl methenamine
- N, N - Dimethylaniline
- N - Methyl aniline
- Benzenamine
Choose the correct order of the basic nature of the above amines.
Write a short note on Ammonolysis.
