Advertisements
Advertisements
प्रश्न
How the following conversion can be carried out?
Ethyl chloride to propanoic acid
उत्तर
\[\ce{\underset{Ethyl chloride}{CH3CH2Cl} ->[KCN/EtOH-H2O][nucleophilic substitution] \underset{Propanenitrile}{CH3CH2CN} ->[H^+/H2O][hydrolysis] \underset{Propanoic acid}{CH3CH2COOH}}\]
APPEARS IN
संबंधित प्रश्न
Write the structure of an isomer of compound C4H9Br which is most reactive towards SN1 reaction
Write the structure of the major product in each of the following reaction :
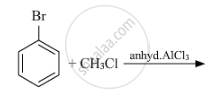
Write the isomers of the compound having the formula C4H9Br.
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2Br + KCN ->[aq.ethanol]}\]
C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Given reasons: The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Which would undergo SN2 reaction faster in the following pair and why ?
CH3 – CH2 – Br and CH3 – CH2 – I
Arrange the following organic compounds in descending order of their reactivity towards SN1 reaction.
C6H5CH2Br, C6H5CH(C6H5)Br, C6H5CH(CH3)Br, C6H5C(CH3)(C6H5)Br
Which one is most reactive towards SN1 reaction?
SN2 mechanism proceeds through intervention of ____________.
Which among MeX, RCH2X, R2CHX and R3CX is most reactive towards SN2 reaction?
Which of the following is a chiral compound?
Identify X and Y in the following sequence:
\[\ce{C2H5Br ->[X] Product ->[Y] C3H7NH2}\]
Which of the following compounds will give a racemic mixture on nucleophilic substitution by OH ion?
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
Which of the following statements are correct about the kinetics of this reaction?
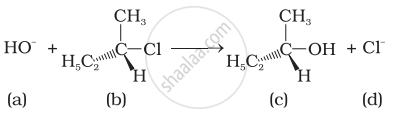
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on concentration of both (a) and (b).
(iii) Molecularity of reaction is one.
(iv) Molecularity of reaction is two.
How do polar solvents help in the first step in SN1 mechanism?
Racemisation occurs in ______.
Discuss the mechanism of alkaline hydrolysis of methyl bromide.
Explain why Grignard reagents should be prepared under anhydrous conditions.
