Advertisements
Advertisements
प्रश्न
Oxygen is filled in a closed metal jar of volume 1.0 × 10−3 m3 at a pressure of 1.5 × 105Pa and temperature 400 K. The jar has a small leak in it. The atmospheric pressure is 1.0 × 105 Pa and the atmospheric temperature is 300 K. Find the mass of the gas that leaks out by the time the pressure and the temperature inside the jar equalise with the surrounding.
उत्तर
Here,
V1 = 1.0 × 10-3 m3
T1 = 400K
P1 = 1.5 × 105 Pa
P2 = 1.0 × 105 Pa
T2 = 300
M = 32 g
Number of moles in the jar before \[n_1 =\frac{P_1 V_1}{R T_1} \]
Volume of the gas when pressure becomes equal to external pressure is given by
\[ \frac{P_1 V_1}{T_1} = \frac{P_2 V_2}{T_2} \]
\[ \Rightarrow V_2 = \frac{P_1 V_1 T_2}{P_2 T_1} \]
\[ \Rightarrow V_2 = \frac{1.5 \times {10}^5 \times 1.0 \times {10}^{-3} \times 300}{1.0 \times {10}^5 \times 400} = 1.125 \times {10}^{-3 }\]
Net volume of leaked gas = V2 - V1
= 1.125 × 10-3 - 1.0 × 10-3
= 1.25 × 10-4 m3
Let n2 be the number of moles of leaked gas. Applying equation of state on this amount of gas, we get
\[ n_2 = \frac{P_2 V_2}{R T_2} = \frac{1.0 \times {10}^5 \times 1.25 \times {10}^{-4}}{8.3 \times 300} = 0.005 \]
Mass of leaked gas = 32 × 0.005 = 0.16 g
APPEARS IN
संबंधित प्रश्न
Molar volume is the volume occupied by 1 mol of any (ideal) gas at standard temperature and pressure (STP: 1 atmospheric pressure, 0 °C). Show that it is 22.4 litres
Estimate the average thermal energy of a helium atom at the temperature of 10 million Kelvin (the typical core temperature in the case of a star).
What do you understand by gas?
Give reasons for the following:
Gas fills the vessel completely in which it is kept.
Choose the correct answer:
The graph of PV vs P for gas is
Name or state the following:
An equation used in chemical calculations which gives a simultaneous effect of changes of temperature and pressure on the volume of a given mass of dry gas
Name or state the following:
The absolute temperature value corresponding to 35°C.
Give reason for the following:
Temperature remaining constant the product of the vol. & the press, of a given mass of dry gas is a constant.
Give reason for the following:
Volumes of gases are converted into s.t.p. conditions and then compared.
Gases exert pressure on the walls of the container because the gas molecules ______
Estimate the average thermal energy of a helium atom at room temperature (27 °C).
Estimate the average thermal energy of a helium atom at the temperature on the surface of the Sun (6000 K).
Which of the following diagrams (Figure) depicts ideal gas behaviour?
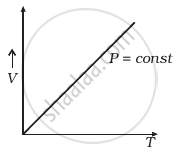 (a) |
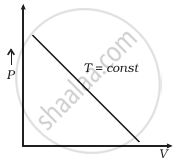 (b) |
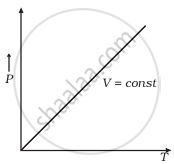 (c) |
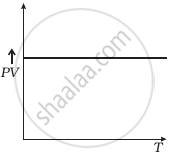 (d) |
Cooking gas containers are kept in a lorry moving with uniform speed. The temperature of the gas molecules inside will ______.
P ∝ T at constant volume is the statement of ______.
Two tanks of equal volume contain equal mass of oxygen and nitrogen at 127°C. Find the ratio of pressure in two tanks.
