Advertisements
Advertisements
प्रश्न
Visible light has wavelengths in the range of 400 nm to 780 nm. Calculate the range of energy of the photons of visible light.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
उत्तर
Given :
Range of wavelengths, `λ_1` = 400 nm to `λ_2` = 780 nm
Planck's constant, `h = 6.63 xx 10^-34 "Js"`
Speed of light, `c = 3 xx 10^8 "m/s"`
Energy of photon,
`E = hv`
`v = c/λ`
`therefore E = hv = (hc)/λ`
Energy `(E_1)` of a photon of wavelength `(λ_1)` :
`E_1 = (hc)/λ_1`
= `(6.63 xx 10^-34 xx 3 xx 10^8)/(400 xx 10^-9)`
= `(6.63 xx 3)/4 xx 10^-9`
= `4.97725 xx 10^-19`
= `5 xx 10^-19 "J"`
Energy `(E_2)` of a photon of wavelength `(λ_2)` :
`E_2 = (6.63 xx 3)/7.8 xx 10^-19`
= `2.55 xx 10^-9 "J"`
So, the range of energy is `2.55 xx 10^-19 "J"` to `5 xx 10^-19 "J"` .
APPEARS IN
संबंधित प्रश्न
(a) A monoenergetic electron beam with electron speed of 5.20 × 106 m s−1 is subject to a magnetic field of 1.30 × 10−4 T normal to the beam velocity. What is the a radius of the circle traced by the beam, given e/m for electron equals 1.76 × 1011 C kg−1?
(b) Is the formula you employ in (a) valid for calculating the radius of the path of a 20 MeV electron beam? If not, in what way is it modified?
Is the formula you employ in (a) valid for calculating radius of the path of a 20 MeV electron beam? If not, in what way is it modified?
What is so special about the combination e/m? Why do we not simply talk of e and m separately?
If light of wavelength 412.5 nm is incident on each of the metals given below, which ones will show photoelectric emission and why?
| Metal | Work Function (eV) |
| Na | 1.92 |
| K | 2.15 |
| Ca | 3.20 |
| Mo | 4.17 |
Two neutral particles are kept 1 m apart. Suppose by some mechanism some charge is transferred from one particle to the other and the electric potential energy lost is completely converted into a photon. Calculate the longest and the next smaller wavelength of the photon possible.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
In an experiment on photoelectric effect, light of wavelength 400 nm is incident on a cesium plate at the rate of 5.0 W. The potential of the collector plate is made sufficiently positive with respect to the emitter, so that the current reaches its saturation value. Assuming that on average, one out of every 106 photons is able to eject a photoelectron, find the photocurrent in the circuit.
A light beam of wavelength 400 nm is incident on a metal plate of work function 2.2 eV. (a) A particular electron absorbs a photon and makes two collisions before coming out of the metal. Assuming that 10% of the extra energy is lost to the metal in each collision, find the kinetic energy of this electron as it comes out of the metal. (b) Under the same assumptions, find the maximum number of collisions the electron can suffer before it becomes unable to come out of the metal.
In the arrangement shown in the figure, y = 1.0 mm, d = 0.24 mm and D = 1.2 m. The work function of the material of the emitter is 2.2 eV. Find the stopping potential V needed to stop the photocurrent.
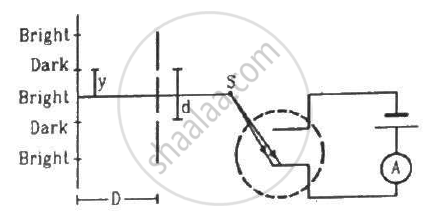
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
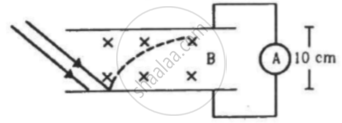
In an experiment on photoelectric effect, the emitter and the collector plates are placed at a separation of 10 cm and are connected through an ammeter without any cell. A magnetic field B exists parallel to the plates. The work function of the emitter is 2.39 eV and the light incident on it has wavelengths between 400 nm and 600 nm. Find the minimum value of B for which the current registered by the ammeter is zero. Neglect any effect of space charge.
The stopping potential in an experiment on photoelectric effect is 1.5V. What is the maximum kinetic energy of the photoelectrons emitted? Calculate in Joules.
Answer the following question.
Why is the wave theory of electromagnetic radiation not able to explain the photoelectric effect? How does a photon picture resolve this problem?
In Photoelectric effect ______.
In the experimental set up for studying photoelectric effect, if keeping the frequency of the incident radiation and the accelerating potential fixed, the intensity of light is varied, then ______.
When a beam of 10.6 eV photons of intensity 2.0 W/m2 falls on a platinum surface of area 1.0 × 10-4 m2, only 53% of the incident photons eject photoelectrons. The number of photoelectrons emitted per second is ______.
The electromagnetic theory of light failed to explain ______.
Cathode rays can be deflected by
