Advertisements
Advertisements
प्रश्न
What is the energy of a hydrogen atom in the first excited state if the potential energy is taken to be zero in the ground state?
उत्तर
In ground state, the potential energy of a hydrogen atom is zero.
An electron is bound to the nucleus with an energy of 13.6 eV.
Therefore, we have to give 13.6 eV energy to move the electron from the nucleus.
Let us calculate the excitation energy required to take an atom from the ground state (n= 1) to the first excited state (n = 2).
`E = 13.6 xx(1/n_1^2 - 1/n_2^2) eV`
Therefore, the excitation energy is given by
`E =13.6xx (1/1^2 - 1/2^2) eV`
`E = 13.6 xx 3/4 eV = 10.2 eV`
Energy of 10.2 eV is needed to take an atom from the ground state to the first excited state.
∴ Total energy of an atom in the first excitation state = 13.6 eV + 10.2 eV = 23.8 eV
APPEARS IN
संबंधित प्रश्न
A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. What series of wavelengths will be emitted?
Find the wavelength of the electron orbiting in the first excited state in hydrogen atom.
In which of the following systems will the radius of the first orbit (n = 1) be minimum?
Which of the following curves may represent the speed of the electron in a hydrogen atom as a function of trincipal quantum number n?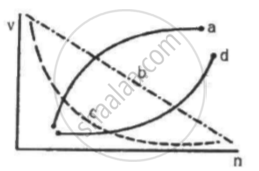
A hydrogen atom in ground state absorbs 10.2 eV of energy. The orbital angular momentum of the electron is increased by
Which of the following products in a hydrogen atom are independent of the principal quantum number n? The symbols have their usual meanings.
(a) vn
(b) Er
(c) En
(d) vr
Let An be the area enclosed by the nth orbit in a hydrogen atom. The graph of ln (An/A1) against ln(n)
(a) will pass through the origin
(b) will be a straight line with slope 4
(c) will be a monotonically increasing nonlinear curve
(d) will be a circle
Calculate the smallest wavelength of radiation that may be emitted by (a) hydrogen, (b) He+ and (c) Li++.
A group of hydrogen atoms are prepared in n = 4 states. List the wavelength that are emitted as the atoms make transitions and return to n = 2 states.
A hydrogen atom in a state having a binding energy of 0.85 eV makes transition to a state with excitation energy 10.2 e.V (a) Identify the quantum numbers n of the upper and the lower energy states involved in the transition. (b) Find the wavelength of the emitted radiation.
A hydrogen atom in state n = 6 makes two successive transitions and reaches the ground state. In the first transition a photon of 1.13 eV is emitted. (a) Find the energy of the photon emitted in the second transition (b) What is the value of n in the intermediate state?
Find the temperature at which the average thermal kinetic energy is equal to the energy needed to take a hydrogen atom from its ground state to n = 3 state. Hydrogen can now emit red light of wavelength 653.1 nm. Because of Maxwellian distribution of speeds, a hydrogen sample emits red light at temperatures much lower than that obtained from this problem. Assume that hydrogen molecules dissociate into atoms.
Electrons are emitted from an electron gun at almost zero velocity and are accelerated by an electric field E through a distance of 1.0 m. The electrons are now scattered by an atomic hydrogen sample in ground state. What should be the minimum value of E so that red light of wavelength 656.3 nm may be emitted by the hydrogen?
A hydrogen atom moving at speed υ collides with another hydrogen atom kept at rest. Find the minimum value of υ for which one of the atoms may get ionized.
The mass of a hydrogen atom = 1.67 × 10−27 kg.
When a photon is emitted from an atom, the atom recoils. The kinetic energy of recoil and the energy of the photon come from the difference in energies between the states involved in the transition. Suppose, a hydrogen atom changes its state from n = 3 to n = 2. Calculate the fractional change in the wavelength of light emitted, due to the recoil.
Consider an excited hydrogen atom in state n moving with a velocity υ(ν<<c). It emits a photon in the direction of its motion and changes its state to a lower state m. Apply momentum and energy conservation principles to calculate the frequency ν of the emitted radiation. Compare this with the frequency ν0 emitted if the atom were at rest.
Let En = `(-1)/(8ε_0^2) (me^4)/(n^2h^2)` be the energy of the nth level of H-atom. If all the H-atoms are in the ground state and radiation of frequency (E2 - E1)/h falls on it ______.
- it will not be absorbed at all.
- some of atoms will move to the first excited state.
- all atoms will be excited to the n = 2 state.
- no atoms will make a transition to the n = 3 state.
Positronium is just like a H-atom with the proton replaced by the positively charged anti-particle of the electron (called the positron which is as massive as the electron). What would be the ground state energy of positronium?
A hydrogen atom makes a transition from n = 5 to n = 1 orbit. The wavelength of photon emitted is λ. The wavelength of photon emitted when it makes a transition from n = 5 to n = 2 orbit is ______.
