Advertisements
Advertisements
प्रश्न
Write information about the given atomic numbers in the table. 10, 20, 7.
| Atomic Number | Electronic configuration | Group | Period | Element |
| 10 | ||||
| 20 | ||||
| 7 |
उत्तर
| Atomic Number | Electronic configuration | Group | Period | Element |
| 10 | 2, 8 | 18 | 2 | Neon (Ne) |
| 20 | 2, 8, 8, 2 | 2 | 4 | Calcium (Ca) |
| 7 | 2, 5 | 15 | 2 | Nitrogen (N) |
APPEARS IN
संबंधित प्रश्न
What do you understand by atomic size? State its unit?
Give the trends in atomic size on moving across the period left to right.
Why is the size of neon greater than fluorine?
Why is the size of sodium is greater than magnesium?
Which is greater in size an atom or a cation?
Which is greater in size Fe2+ or Fe3+?
Fill in the blank:
On moving across a period from right to left in the periodic table, the atomic size of the atom ___________.
Give reasons for the following:
The size of the anion is greater than the size of the parent atom.
Arrange the following in order of increasing radii:
CI- , CI
Write the name and symbol of the element from the description.
The atom having the smallest atomic mass.
Fill in the blank by selecting the correct word from the bracket.
If an element has seven electrons in its outermost shell then it is likely to have the _____ atomic size among all the elements in the same period.
On moving from left to right in a periodic table, the size of the atom _______.
_______ is the distance between the nucleus of the atom and its outermost shell.
Which of the following is the correct order of atomic size?
Elements have been arranged in the following sequence on the basis of their increasing atomic masses.
| F, | Na, | Mg, | Al, | Si, | P, | S, | Cl, | Ar, | K |
- Pick two sets of elements which have similar properties.
- The given sequence represents which law of classification of elements?
Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the bond formed?
- In below ladder symbols of elements are jumbled up. Rearrange these symbols of elements in the increasing order of their atomic number in the Periodic Table.
- Arrange them in the order of their group also.
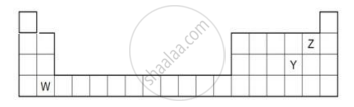
The diagram below shows part of the periodic table.
- Which elements would react together to form covalent compounds?
- Between the two elements W and Z, which will have a bigger atomic radius? Why?