Advertisements
Advertisements
प्रश्न
A small metal plate (work function φ) is kept at a distance d from a singly-ionised, fixed ion. A monochromatic light beam is incident on the metal plate and photoelectrons are emitted. Find the maximum wavelength of the light beam, so that some of the photoelectrons may go round the ion along a circle.
उत्तर
From Einstein's photoelectric equation ,
`eV_0 = (hc)/lambda - phi`
⇒ `V_0 = ((hc)/lambda - phi)1/e`
Here, V0 = stopping potential
h = Planck's constant
c = speed of light
`phi ` = work function
The particle will move in a circle when the stopping potential is equal to the potential due to the singly charged ion at that point so that the particle gets the required centripetal force for its circular motion.
`⇒ (Ke)/(2d) = ((hc)/lambda - phi)1/e`
`⇒ (Ke^2)/(2d) = (hc)/lambda - phi`
`⇒ (hc)/lambda = (Ke^2)/(2d) + phi = (Ke^2+2dphi)/(2d)`
`⇒ lambda = ((hc)(2d))/(ke^2+2dphi)`
`⇒ lambda = (2hdc)/(1/(4pi∈_0)e^2+2dphi`
`⇒ lambda = (8pi∈_0hcd)/(e^2+8pi∈_0dphi)`
APPEARS IN
संबंधित प्रश्न
In an experiment on the photoelectric effect, the slope of the cut-off voltage versus the frequency of incident light is found to be 4.12 × 10−15 Vs. Calculate the value of Planck’s constant.
The work function for a certain metal is 4.2 eV. Will this metal give photoelectric emission for incident radiation of wavelength 330 nm?
Plot a graph showing the variation of photoelectric current with collector plate potential at a given frequency but for two different intensities I1 and I2, where I2 > I1.
Write Einstein’s photoelectric equation?
point out any two characteristic properties of photons on which Einstein’s photoelectric equation is based ?
Briefly explain the three observed features which can be explained by Einstein’s photoelectric equation.
Define the terms (i) ‘cut-off voltage’ and (ii) ‘threshold frequency’ in relation to the phenomenon of photoelectric effect.
Using Einstein’s photoelectric equation shows how the cut-off voltage and threshold frequency for a given photosensitive material can be determined with the help of a suitable plot/graph.
Is p − E/c valid for electrons?
The electric field at a point associated with a light wave is `E = (100 "Vm"^-1) sin [(3.0 xx 10^15 "s"^-1)t] sin [(6.0 xx 10^15 "s"^-1)t]`.If this light falls on a metal surface with a work function of 2.0 eV, what will be the maximum kinetic energy of the photoelectrons?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Choose the correct answer from given options
Photons of frequency v are incident on the surface of two metals A and B of threshold frequency 3/4 v and 2/3 v, respectively. The ratio of maximum kinetic energy of electrons emitted from A to that from B is
Each photon has the same speed but different ______.
The minimum energy required to remove an electron is called ______.
The wavelength of a photon needed to remove a proton from a nucleus which is bound to the nucleus with 1 MeV energy is nearly ______.
- In the explanation of photo electric effect, we assume one photon of frequency ν collides with an electron and transfers its energy. This leads to the equation for the maximum energy Emax of the emitted electron as Emax = hν – φ0 where φ0 is the work function of the metal. If an electron absorbs 2 photons (each of frequency ν) what will be the maximum energy for the emitted electron?
- Why is this fact (two photon absorption) not taken into consideration in our discussion of the stopping potential?
There are materials which absorb photons of shorter wavelength and emit photons of longer wavelength. Can there be stable substances which absorb photons of larger wavelength and emit light of shorter wavelength.
A student performs an experiment on photoelectric effect, using two materials A and B. A plot of Vstop vs ν is given in Figure.
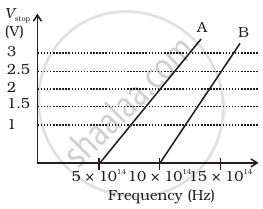
- Which material A or B has a higher work function?
- Given the electric charge of an electron = 1.6 × 10–19 C, find the value of h obtained from the experiment for both A and B.
Comment on whether it is consistent with Einstein’s theory:
The photon emitted during the de-excitation from the first excited level to the ground state of a hydrogen atom is used to irradiate a photocathode in which the stopping potential is 5 V. Calculate the work function of the cathode used.
