Advertisements
Advertisements
प्रश्न
Calculate the change in internal energy of a gas kept in a rigid container when 100 J of heat is supplied to it.
उत्तर
Given:-
Heat supplied to the system,
ΔQ = 100 J
Using the first law of thermodynamics, we get
\[∆ U = ∆ Q - ∆ W\]
Since the container is rigid, initial volume of the system is equal to the final volume of the system. Thus,
\[∆V=V_f - V_i=0\]
∆W = P
∆V = 0
∆U = ∆Q = 100J
We see that heat supplied to the system is used up in raising the internal energy of the system.
APPEARS IN
संबंधित प्रश्न
Choose the correct option.
Which of the following is an example of the first law of thermodynamics?
Two moles of an ideal gas is expanded isothermally and reversibly at 300 K from 1 L to 10 L. The enthalpy change in kJ is ______.
When heat energy of 2000 joules is supplied to a gas at constant pressure 2.1 x 105 N/m2, there is an increase in its volume equal to 2.5 x 10-3 m3. The increase in internal energy of the gas in joules is ____________.
A gas performs 0.320 kJ work on surrounding and absorbs 120 J of heat from the surrounding. Hence, change in internal energy is ______.
In a given process for an ideal gas, dW = 0 and dQ < 0. Then for the gas ____________.
120 J of heat is added to a gaseous system, whose internal energy change is 60 J, then the amount of external work done is ____________.
Calculate the amount of work done during isothermal expansion of a gas from a volume of 4 dm3 to 6 dm3 against a constant external pressure of 3 atmosphere?
Change in internal energy, when 4 KJ of work is done on the system and 1 KJ heat is given out by the system, is:
An ideal gas undergoes cyclic process ABCDA as shown in given P-V diagram (figure). The amount of work done by the gas is ______.

A cycle followed by an engine (made of one mole of an ideal gas in a cylinder with a piston) is shown in figure. Find heat exchanged by the engine, with the surroundings for each section of the cycle. (Cv = (3/2)R)
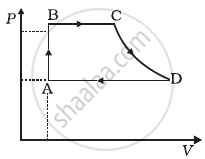
- AB : constant volume
- BC : constant pressure
- CD : adiabatic
- DA : constant pressure
Consider one mole of perfect gas in a cylinder of unit cross section with a piston attached (figure). A spring (spring constant k) is attached (unstretched length L) to the piston and to the bottom of the cylinder. Initially the spring is unstretched and the gas is in equilibrium. A certain amount of heat Q is supplied to the gas causing an increase of volume from V0 to V1.
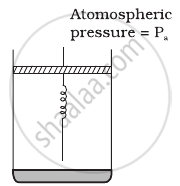
- What is the initial pressure of the system?
- What is the final pressure of the system?
- Using the first law of thermodynamics, write down a relation between Q, Pa, V, Vo and k.
If one mole of monoatomic gas `(gamma=5/3)` is mixed with one mole of diatomic gas `(gamma=7/5)`, the value of γ for the mixture is ______.
ΔU = 0 is true for ______.
What work will be done, when 3 moles of an ideal gas are compressed to half the initial volume at a constant temperature of 300 K?
An ideal gas (γ = 1.5) is expanded adiabatically. How many times has the gas had to be expanded to reduce the root mean square velocity of molecules two times?
In an adiabatic process, ______.
What is true for an adiabatic process?
Show that the heat absorbed at constant pressure is equal to the change in enthalpy of the system.
Calculate work done when 2 moles of ideal gas expands by 5 dm3 isothermally at pressure 1.2 bar.
