Advertisements
Advertisements
प्रश्न
Draw geometric isomers of the following complex.
Geometrical isomers of Pt(NH3)2Cl2
उत्तर
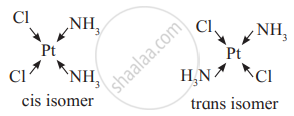
APPEARS IN
संबंधित प्रश्न
Answer the following in one or two sentences.
Predict whether the [Cr(en)2(H2O)2]3+ complex is chiral. Write the structure of its enantiomer.
Answer in brief.
What are ionization isomers ? Give an example.
Draw isomers of the following.
\[\ce{Pt(NH3)2ClNO2}\]
Answer the following question.
Draw geometric isomers and enantiomers of the following complex.
[Pt(en)3]4⊕
Draw structure of cis isomer of [Co(NH3)4Cl2]+
Draw all possible geometrical isomers of the complex \[\ce{[Co(en)2Cl2]^+}\] and identify the optically active isomer.
What is linkage isomerism? Explain with an example.
What are hydrate isomers? Explain with an example.
The term anomers of glucose refer to ____________.
Which would exhibit coordination isomerism?
Which of the following is NOT a pair of structural isomers?
Identify the CORRECT statement about the following complex of platinum.
[PtCl2(en)2]2+
The number of geometrical isomers of [CrCl2(en)2]+ is ____________.
Which of the following does NOT show optical isomerism?
The formula of two complexes X and Y of chromium are given below:
\[\ce{\underset{(X)}{[Cr(H2O)6]Cl3}}\] and \[\ce{\underset{(Y)}{[Cr(H2O)5Cl]Cl2.H2O}}\]
X and Y are examples of ____________ isomers.
Which of the following compound show optical isomerism?
The compounds [PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2 constitutes a pair of ______.
Give cis isomer of [Co(NH3)4Cl2]⊕.
Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers: [Pt(NH3)(H2O)Cl2]
Write structures for geometrical isomers of diamminebromochloroplatinum (II).
What are structural isomers or constitutional isomers?
Match the pairs in column I (pairs of isomers) and column II (types of isomers)
| Column I (Pairs of isomers) |
Column II (Types of isomers) |
| (A) [Cr(H2O)5Cl]Cl2.H2O and [Cr(H2O)4Cl2]Cl.2H2O | (i) Ionization isomers |
| (B) [Co(en)2(NO2)2]+ and [Co(en)2(ONO2)]+ | (ii) Hydrate isomers |
| (C) [Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] | (iii) Linkage isomers |
| (D) [Pt(NH3)4Cl2] Br2 and [Pt(NH3)4Br2]Cl2 | (iv) Coordination isomers |
Draw the structure of cis isomers of Pt(NH3)2Cl2.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Cr(H2O)5Cl]Cl2H2O and [Cr(H2O)4Cl2]Cl {.} 2H2O}\]
Three organic compounds A, B and C are non cyclic functional isomers of carbonyl compounds with molecular formula C4H8O. Isomers A and C give positive Tollen’s test while compound B does not give positive Tollen’s test but gives positive iodoform test. Compounds A and B on reduction with Zn amalgam and conc. HCl give the same product.
- Write the structures of the compounds A, B and C.
- Out of the compounds A, B and C, which one will be the least reactive towards addition of HCN.
