Advertisements
Advertisements
प्रश्न
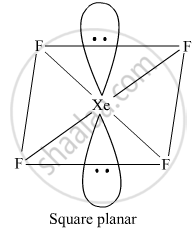
Draw the structure of XeF4.
उत्तर
XeF4
It has a square planar structure. Six electron pairs form an octahedron with two positions occupied by lone pairs.
संबंधित प्रश्न
Draw the structures of the following molecules: XeF4
Complete the equation : XeF2+PF5 →
Balance the following equation: XeF6 + H2O → XeO2F2 + HF
Give the formula and describe the structure of a noble gas species which is isostructural with
`ICI_4^(-)`
Write electronic configuration and two uses of neon. (Z = 10)
Sulfur dioxide reacts with sodium hydroxide solution to form _______.
In which of the following pairs, the two species are isostructural:
Noble gases are named because of their inertness towards reactivity. Identify an incorrect statement about them.
\[\ce{XeF6 + H2O ->[Partial][Hydrolysis] \underline{}\underline{}\underline{}\underline{} + \underline{}\underline{}\underline{}\underline{}}\]
Write two uses of neon.
