Advertisements
Advertisements
प्रश्न
For the past some time, Aarti had been observing some erratic body movement, unsteadiness and lack of coordination in the activities of her sister Radha, who also used to complain of severe headache occasionally. Aarti suggested to her parents to get a medical check-up of Radha. The doctor thoroughly examined Radha and diagnosed that she has a brain tumour.
(a) What, according to you, are the values displayed by Aarti?
(b) How can radioisotopes help a doctor to diagnose brain tumour?
उत्तर
(a) Aarti has displayed awareness and care towards the health of her sister.
(b) During the intake of different elements and compounds, the biological organisms absorb them differently. Also, the exact distribution of the elements and their function in the various parts of organisms cannot be known clearly. For this, a radioisotope is made to enter the organism along with the elements and compounds, whose absorption, functioning and distribution to the brain has to be studied. The radioisotope acts as a tag of label for the element or compound under study. By detecting the radiation emitted by the isotope from the brain, the details regarding the absorption and function of the compounds by the organisms are found out. In this way, radioisotopes help a doctor to diagnose brain tumour.
APPEARS IN
संबंधित प्रश्न
The sequence of stepwise decay of a radioactive nucleus is
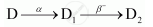
If the atomic number and mass number of D2 are 71 and 176 respectively, what are their corresponding values of D?
The half-life of 226Ra is 1602 y. Calculate the activity of 0.1 g of RaCl2 in which all the radium is in the form of 226Ra. Taken atomic weight of Ra to be 226 g mol−1 and that of Cl to be 35.5 g mol−1.
A vessel of volume 125 cm3 contains tritium (3H, t1/2 = 12.3 y) at 500 kPa and 300 K. Calculate the activity of the gas.
The count rate of nuclear radiation coming from a radiation coming from a radioactive sample containing 128I varies with time as follows.
| Time t (minute): | 0 | 25 | 50 | 75 | 100 |
| Ctount rate R (109 s−1): | 30 | 16 | 8.0 | 3.8 | 2.0 |
(a) Plot In (R0/R) against t. (b) From the slope of the best straight line through the points, find the decay constant λ. (c) Calculate the half-life t1/2.
A human body excretes (removes by waste discharge, sweating, etc.) certain materials by a law similar to radioactivity. If technetium is injected in some form in a human body, the body excretes half the amount in 24 hours. A patient is given an injection containing 99Tc. This isotope is radioactive with a half-life of 6 hours. The activity from the body just after the injection is 6 μCi. How much time will elapse before the activity falls to 3 μCi?
A sample contains a mixture of 108Ag and 110Ag isotopes each having an activity of 8.0 × 108 disintegration per second. 110Ag is known to have larger half-life than 108Ag. The activity A is measured as a function of time and the following data are obtained.
| Time (s) |
Activity (A) (108 disinte- grations s−1) |
Time (s) |
Activity (A 108 disinte-grations s−1) |
| 20 40 60 80 100 |
11.799 9.1680 7.4492 6.2684 5.4115 |
200 300 400 500 |
3.0828 1.8899 1.1671 0.7212 |
(a) Plot ln (A/A0) versus time. (b) See that for large values of time, the plot is nearly linear. Deduce the half-life of 110Ag from this portion of the plot. (c) Use the half-life of 110Ag to calculate the activity corresponding to 108Ag in the first 50 s. (d) Plot In (A/A0) versus time for 108Ag for the first 50 s. (e) Find the half-life of 108Ag.
The half-life of radium is 1550 years. Calculate its disintegration constant (`lambda`) .
Copy and complete the following table for a radioactive element whose half-life is 10 minutes. Assume that you have 30g of this element at t = 0.

Complete the following nuclear reactions :
(i) `"_15^32P -> ` `"_z^AX + bar(e) + bar(v)`
(ii) `"_6^12 C `+`"_6^12C ->` ` "_2^A Y + ` `"_4^2 He`
The half-life of a certain radioactive element is 3.465 days. Find its disintegration constant.
