Advertisements
Advertisements
प्रश्न
For the reaction, \[\ce{A +2B → AB2}\], the order w.r.t. reactant A is 2 and w.r.t. reactant B. What will be change in rate of reaction if the concentration of A is doubled and B is halved?
पर्याय
Increases four times
Decreases four times
Increases two times
No change
उत्तर
Increases four times
Explanation:
Rate = [A]2
If [A] is doubled then Rate = [2A]2 = 4[A]2 = 4 Rate
APPEARS IN
संबंधित प्रश्न
Write molecularity of the following reaction:
2NO(g)+O2(g)→2NO2(g)
For a reaction, \[\ce{A + B -> Product}\]; the rate law is given by, `r = k[A]^(1/2)[B]^2`. What is the order of the reaction?
A reaction is first order in A and second order in B. How is the rate affected on increasing the concentration of B three times?
For a complex reaction:
(i) order of overall reaction is same as molecularity of the slowest step.
(ii) order of overall reaction is less than the molecularity of the slowest step.
(iii) order of overall reaction is greater than molecularity of the slowest step.
(iv) molecularity of the slowest step is never zero or non interger.
Match the graph given in Column I with the order of reaction given in Column II. More than one item in Column I may link to the same item of Column II.
| Column I | Column II | |
| (i) | 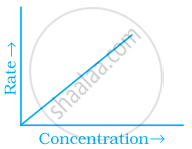 |
|
| (ii) | 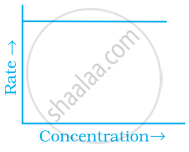 |
(a) 1st order |
| (iii) | 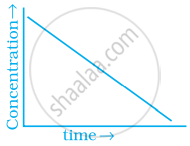 |
(b) Zero-order |
| (iv) | 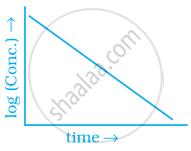 |
For a reaction A + B → products, the rate law is given by: r = `K[A]^(1/2)`. What is the order of reaction?
If the 0.05 molar solution of m+ is replaced by a 0.0025 molar m+ solution, then the magnitude of the cell potential would be
Identify the order of reaction from the following unit for its rate constant:
L mol–1s–1
Read the following passage and answer the questions that follow:
|
The rate of reaction is concerned with decrease in the concentration of reactants or increase in the concentration of products per unit of time. It can be expressed as instantaneous rate at a particular instant of time and average rate over a large interval of time. A number of factors such as temperature, concentration of reactants, catalyst affect the rate of reaction. Mathematical representation of rate of a reaction is given by rate law: Rate = k[A]x [B]y x and y indicate how sensitive the rate is to change in concentration of A and B. Sum of x + y gives the overall order of a reaction. |
- What is the effect of temperature on the rate constant of a reason? [1]
- For a reaction \[\ce{A + B → Product}\], the rate law is given by, Rate = k[A]2 [B]1/2. What is the order of the reaction? [1]
- How order and molecularity are different for complex reactions? [1]
- A first-order reaction has a rate constant 2 × 10–3 s–1. How long will 6 g of this reactant take to reduce to 2 g? [2]
OR
The half-life for radioactive decay of 14C is 6930 years. An archaeological artifact containing wood had only 75% of the 14C found in a living tree. Find the age of the sample.
[log 4 = 0.6021, log 3 = 0.4771, log 2 = 0.3010, log 10 = 1] [2]
On heating compound (A) gives a gas (B) which is constituent of air. The gas when treated with H2 in the presence of catalyst gives another gas (C) which is basic in nature, (A) should not be ______.
