Advertisements
Advertisements
प्रश्न
Give one example of a molecule containing a double covalent bond
उत्तर
A molecule containing a double covalent bond: oxygen molecule 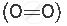
APPEARS IN
संबंधित प्रश्न
Choose the most appropriate answer from the following list of oxides which fit the description.
A covalent oxide of a metalloid.
Buckminsterfullerene is spherical molecule in which 60 carbon atoms are arranged in interlocking hexagonal and pentagonal rings of carbon atoms.
How many hexagons of carbon atoms are present in one molecule of buckminsterfullerene?
Fill in the blank in the following sentence:
Two chlorine atoms combine to form a molecule. The bond between them is known as ..........
Friedrich Wohler converted an inorganic compound into an organic compound in the laboratory.
Write the name and formula of organic compound formed.
Give the formula of the compound that would be formed by the combination of the following pair of elements:
K and H
Explain why, diamond has a high melting point.
What is graphite?
Buckminsterfullerene is an allotropic form of the element:
(a) phoshorus
(b) fluorine
(c) carbon
(d) sulphur
A solid element X has four electrons in the outermost shell of its atom. An allotrope Y of this element is used as a dry lubricant in machinery and also in making pencil leads.
(a) What is element X?
(b) Name the allotrope Y.
(c) State whether allotrope Y is a good conductor or non-conductor of electricity.
(d) Name one use of allotrope Y (other than lubrication and pencil leads)
(e) Name two other allotropes of element X.
Explain the following term with example.
Hetero atom in a carbon compound
Explain the following briefly:
Sodium chloride dissolves in water but carbon tetra chloride is insoluble in water.
Explain the following briefly.
Pure water does not conduct electricity, but on adding sodium chloride to it, it starts conducting electricity.
Explain the following:
Covalent compounds are generally gases or liquids or soft solids.
Acids dissolve in water to produce positively charged ions. Draw the structure of these positive ions.
Name a neutral covalent molecule which contains one lone pair of electrons.
The electronic configuration of N2 is 2, 5. How many electrons in the outer shell of a N atom are not involved in the formation of a nitrogen molecule?
Element A has 2 electrons in its M shell. Element B has atomic number 7.
If the compound formed between A and B is melted and an electric current is passed through the molten compound, the element A will be obtained at the ______ and B at the ______ of the electrolytic cell.
Covalent bonds can be single, double or triple covalent bonds. How many electrons are shared in each? Give an example of each type.
Which of the following statements are usually correct for carbon compounds? These
- are good conductors of electricity
- are poor conductors of electricity
- have strong forces of attraction between their molecules
- do not have strong forces of attraction between their molecules.
In covalent bond formation, the sharing of ______ electrons takes place in their outermost shell.
