Advertisements
Advertisements
प्रश्न
Identify the gas evolved in the following reaction:
\[\ce{MnO2}\] reacts with concentrated \[\ce{HCl}\].
उत्तर
Chlorine
Explanation:
Manganese oxide reacts with cone. \[\ce{HCl}\], releasing greenish colored chlorine and forming manganese chloride.
\[\ce{MnO2 + HCl2 -> MnCl2 + Cl2 + 2H2O}\]
APPEARS IN
संबंधित प्रश्न
Write a balanced chemical equation for the action of hydrochloric acid on sodium bicarbonate.
Identify the gas evolved and give the chemical test in the following cases
Dilute hydrochloric acid reacts with iron (II) sulphide.
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
Write the equation for its preparation mentioning the condition required.
Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following.
- Name the acid used. Why is this particular acid preferred to other acids?
- Give the balanced equation for the reaction.
- Name the drying agent used in drying hydrogen chloride gas.
- Phosphorous pentoxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gas. Why?
- Why is the direct absorption of \[\ce{HCl}\] gas in water not feasible?
- What arrangement is done to dissolve \[\ce{HCl}\] gas in the water?
Name the drying agents used in drying hydrogen chloride gas.
Explain why a solution of hydrogen chloride in water turns blue litmus red and conducts electricity, while a solution of the same gas in toluene:
- has no effect on litmus, and
- does not conduct electricity.
Write the main difference in hydrogen chloride gas and hydrochloric acid.
The given set up in the figure is for the preparation of an acid.
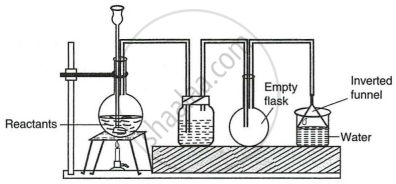
- Name the acid prepared by this method.
- Name the reactants used.
- Why an empty flask is used?
- What is the drying agent used? Why is this drying agent chosen?
- What is the role of the inverted funnel in the arrangement?
What are the important precautions?
Explain, why (or give reasons for)
In the preparation chloride from sodium chloride, the gas can be obtained below 200°C or above. But the lower temperature is preferred.
Explain, why (or give reasons for)
Hydrogen chloride is not collected over water.
What is aqua-regia?
Choose the correct answer from the options given below:
Dilute hydrochloric acid solution cannot be concentrated by boiling beyond
Write balanced equation for the reaction between dilute hydrochloric acid and sodium sulphite.
Write the equation for :
The preparation of hydrogen chloride from sodium chloride and sulphuric acid. State whether the sulphuric acid should be concentrated or dilute.
State the observation for action of dilute hydrochloiric acid or iron (II) sulphate.
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
