Advertisements
Advertisements
प्रश्न
What is aqua-regia?
उत्तर
Aqua regia is a mixture is 3 parts of concentrated HCI and 1 part of concentrated HNO3.
APPEARS IN
संबंधित प्रश्न
Certain blank spaces are left in the following table and these are labelled as A, B, C, D
and E. Identify each of them
| Lab preparation of | Reactants used | Products formed | Drying Agent | Method of collection |
|
| 1 | HCl gas | NaCl + H2SO4 |  |
conc. H2SO4 | 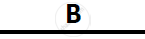 |
| 2 | NH3 gas |
|
Mg(OH)2 NH3 |
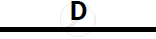 |
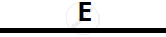 |
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
State a safety precaution you would take during the preparation of hydrochloric acid.
Give the balanced equation for the laboratory preparation of hydrogen chloride gas reaction.
Name the drying agents phosphorus pentoxide and calcium oxide are good drying agent but they cannot be used to dry hydrogen chloride gas. Why?
Explain why when the stopper of a bottle full of hydrogen chloride gas is opened, there are fumes in the air.
Explain why dry hydrogen chloride gas does not affect a dry strip of blue litmus paper but it turns red in the presence of a drop of water.
What are the important precautions?
Explain, why (or give reasons for)
In the preparation chloride from sodium chloride, the gas can be obtained below 200°C or above. But the lower temperature is preferred.
Mention the reaction condition and give balanced equation to obtain: Cl2 gas from HCI acid.
Choose the correct answer from the options given below:
Dilute hydrochloric acid solution cannot be concentrated by boiling beyond
Write the equation for :
The preparation of hydrogen chloride from sodium chloride and sulphuric acid. State whether the sulphuric acid should be concentrated or dilute.
State the observation for action of dilute hydrochloiric acid or iron (II) sulphate.
Give one test to distinguish between the following pair of chemicals.
Sodium nitrate solution and sodium chloride solution.
Answer the following question related to the laboratory preparation of the hydrogen chloride gas:
Why is sodium chloride preferred to other metallic chlorides?
Answer the following question related to the laboratory preparation of the hydrogen chloride gas:
Write the chemical equation.
In the laboratory preparation, HCl gas is dried by passing through ______.
State a relevant reason for the following:
Hydrogen chloride gas cannot be dried over quick lime.
Identify the gas evolved in the following reaction:
\[\ce{MnO2}\] reacts with concentrated \[\ce{HCl}\].
