Advertisements
Advertisements
प्रश्न
In the cubic close packing, the unit cell has ______.
पर्याय
4 tetrahedral voids each of which is shared by four adjacent unit cells.
4 tetrahedral voids within the unit cell.
8 tetrahedral voids each of the which is shared by four adjacent unit cells.
8 tetrahedral voids within the unit cells.
उत्तर
In the cubic close packing, the unit cell has 8 tetrahedral voids within the unit cells.
Explanation:
Eight tetrahedral voids per fee unit cell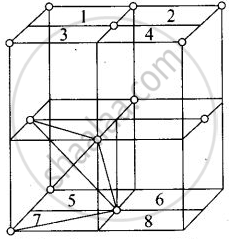
Each cube represented by numeric 1, 2, 3, 4, 5, 6, 7, 8 contains one tetrahedral void.
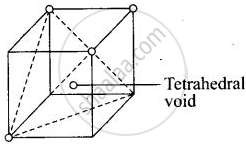
Each cube contains one tetrahedral void at its body centre as shown above
APPEARS IN
संबंधित प्रश्न
How many tetrahedral voids can exist per unit cell in a hexagonal close packing sphere?
Total number of voids in 0.5 mole of a compound which forms hexagonal close packed structure is ____________.
Percentages of free space in cubic close packed structure and in the body-centered packed structure are respectively:
In the hexagonal close-packed structure of a metallic lattice, the number of nearest neighbours of a metallic atom is ____________.
If Germanium crystallises in the same way as diamond, then which of the following statement is not correct?
The coordination number of Y will be in the XY types of crystal:
Which of the following is not true about the voids formed in 3 dimensional hexagonal close packed structure?
(i) A tetrahedral void is formed when a sphere of the second layer is present above triangular void in the first layer.
(ii) All the triangular voids are not covered by the spheres of the second layer.
(iii) Tetrahedral voids are formed when the triangular voids in the second layer lie above the triangular voids in the first layer and the triangular shapes of these voids do not overlap.
(iv) Octahedral voids are formed when the triangular voids in the second layer exactly overlap with similar voids in the first layer.
Show that in a cubic close packed structure, eight tetrahedral voids are present per unit cell.
A solid compound XY has Nacl structure. If the radium of cation (X+) is 100 pm, the radium of anion (r–) will be:-
In which of the following structures coordination number for cations and anions in the packed structure will be same?
